Monogram Technologies Announces World’s First Fully Autonomous Saw-Based Robotic Knee Replacement Surgery
Monogram Technologies (NASDAQ: MGRM) has achieved a historic milestone by completing the world's first fully autonomous saw-based robotic knee replacement surgery on a live patient. The groundbreaking procedure was performed on July 26, 2025, at Krishna Shalby Hospital in Ahmedabad, India, using Monogram's mBôs TKA System.
The surgery is part of a 102-patient multi-center clinical trial in India, approved by CDSCO, evaluating the system's safety and effectiveness. The procedure utilized the Consensus CKS implant, which is substantially equivalent to Monogram's mPress implants. This achievement follows the recent FDA 510(k) clearance for Monogram's semi-autonomous TKA System and comes amid Zimmer Biomet's (NYSE: ZBH) pending acquisition of Monogram.
Monogram Technologies (NASDAQ: MGRM) ha raggiunto un traguardo storico completando il primo intervento chirurgico al mondo di sostituzione del ginocchio completamente autonomo con sega robotica su un paziente in vita. La procedura innovativa è stata eseguita il 26 luglio 2025 presso l'ospedale Krishna Shalby di Ahmedabad, in India, utilizzando il sistema mBôs TKA di Monogram.
L'intervento fa parte di uno studio clinico multicentrico su 102 pazienti in India, approvato dal CDSCO, che valuta la sicurezza e l'efficacia del sistema. La procedura ha impiegato l'impianto Consensus CKS, sostanzialmente equivalente agli impianti mPress di Monogram. Questo risultato segue la recente approvazione FDA 510(k) per il sistema TKA semi-autonomo di Monogram e arriva in un momento in cui Zimmer Biomet (NYSE: ZBH) sta per acquisire Monogram.
Monogram Technologies (NASDAQ: MGRM) ha alcanzado un hito histórico al completar la primera cirugía de reemplazo de rodilla totalmente autónoma con sierra robótica en un paciente vivo. El procedimiento innovador se realizó el 26 de julio de 2025 en el Hospital Krishna Shalby en Ahmedabad, India, utilizando el sistema mBôs TKA de Monogram.
La cirugía forma parte de un ensayo clínico multicéntrico con 102 pacientes en India, aprobado por CDSCO, que evalúa la seguridad y efectividad del sistema. El procedimiento utilizó el implante Consensus CKS, que es sustancialmente equivalente a los implantes mPress de Monogram. Este logro sigue a la reciente aprobación FDA 510(k) para el sistema TKA semiautónomo de Monogram y se produce en medio de la adquisición pendiente de Monogram por parte de Zimmer Biomet (NYSE: ZBH).
Monogram Technologies (NASDAQ: MGRM)�� 생체 환자에게 세계 최초�� 완전 자율�� �� 기반 로봇 무릎 교체 수술�� 성공적으�� 완료하며 역사적인 이정표를 세웠습니��. �� 획기적인 수술은 2025�� 7�� 26�� 인도 아메다바드의 Krishna Shalby 병원에서 Monogram�� mBôs TKA 시스���� 사용하여 진행되었습니��.
�� 수술은 CDSCO�� 승인�� 받은 인도 �� 102�� 환자 다기관 임상시험�� 일환으로, 시스템의 안전성과 효과�� 평가합니��. 수술에는 Monogram�� mPress 임플란트와 실질적으�� 동등�� Consensus CKS 임플란트가 사용되었습니��. 이번 성과�� 최근 Monogram�� 반자�� TKA 시스템에 대�� FDA 510(k) 승인 이후 이루어졌으며, Zimmer Biomet (NYSE: ZBH)�� Monogram�� 인수하는 과정 중에 이루어졌습니��.
Monogram Technologies (NASDAQ : MGRM) a franchi une étape historique en réalisant la première chirurgie de remplacement du genou entièrement autonome, basée sur une scie robotisée, sur un patient vivant. Cette procédure révolutionnaire a été effectuée le 26 juillet 2025 à l'hôpital Krishna Shalby d'Ahmedabad, en Inde, en utilisant le système mBôs TKA de Monogram.
Cette chirurgie fait partie d'un essai clinique multicentrique portant sur 102 patients en Inde, approuvé par le CDSCO, évaluant la sécurité et l'efficacité du système. La procédure a utilisé l'implant Consensus CKS, qui est substantiellement équivalent aux implants mPress de Monogram. Cette réussite fait suite à la récente validation FDA 510(k) du système TKA semi-autonome de Monogram et intervient alors que Zimmer Biomet (NYSE : ZBH) est en cours d'acquisition de Monogram.
Monogram Technologies (NASDAQ: MGRM) hat einen historischen Meilenstein erreicht, indem es die weltweit erste vollständig autonome, sägebasierte robotergestützte Kniegelenkersatzoperation an einem lebenden Patienten erfolgreich durchgeführt hat. Der bahnbrechende Eingriff fand am 26. Juli 2025 im Krishna Shalby Hospital in Ahmedabad, Indien, unter Verwendung des mBôs TKA Systems von Monogram statt.
Die Operation ist Teil einer multizentrischen klinischen Studie mit 102 Patienten in Indien, die von der CDSCO genehmigt wurde und die Sicherheit und Wirksamkeit des Systems bewertet. Dabei wurde das Consensus CKS Implantat verwendet, das dem mPress-Implantat von Monogram im Wesentlichen entspricht. Dieser Erfolg folgt auf die kürzliche FDA 510(k)-Zulassung des halbautonomen TKA-Systems von Monogram und erfolgt im Zuge der bevorstehenden Übernahme von Monogram durch Zimmer Biomet (NYSE: ZBH).
- None.
- Clinical trial results and long-term effectiveness data still pending
- Full regulatory approvals for autonomous system not yet obtained globally
Insights
Monogram's autonomous knee replacement surgery milestone advances its acquisition value for Zimmer Biomet while validating its revolutionary robotic technology.
Monogram Technologies has achieved a groundbreaking milestone with the world's first fully autonomous saw-based robotic knee replacement surgery on a live patient. This watershed moment demonstrates that the company's mBôs TKA System can execute precise surgical plans with minimal human intervention �� a quantum leap beyond existing semi-autonomous systems that require continuous surgeon control.
The procedure's success in India, part of a 102-patient clinical trial across multiple centers, validates the safety and effectiveness of Monogram's technology. What's particularly significant is that this comes shortly after the FDA granted 510(k) clearance for Monogram's semi-autonomous TKA System in the US market, creating a clear regulatory pathway.
The timing couldn't be more strategic given Zimmer Biomet's pending acquisition of Monogram. This successful procedure strengthens Zimmer Biomet's position in the
The partnership with Shalby Limited, one of the world's largest orthopedic hospital groups, provides an ideal clinical validation platform with high-volume surgical centers. What separates this technology from existing systems is the autonomous execution of the complex bone-cutting procedure �� the most technically challenging and highest-risk portion of knee replacement surgery.
This development positions Monogram's technology to potentially reduce procedure time, improve surgical precision, and standardize outcomes across surgeons with varying experience levels �� addressing the three most critical challenges in joint replacement procedures today.
The Procedure Utilized Monogram’s mBôs TKA System Performed in India with Shalby Hospitals
Groundbreaking Procedure Delivers the World’s First Autonomous Saw-Based Robotic TKA Surgery on a Live Patient
AUSTIN, Texas, July 29, 2025 (GLOBE NEWSWIRE) -- Monogram Technologies Inc. (NASDAQ: MGRM) ("Monogram" or the "Company"), an AI-driven robotics company revolutionizing orthopedic surgery, today announced that on Saturday, July 26, 2025, Monogram completed the first ever fully autonomous saw-based robotic knee replacement procedure on a live patient.
The procedure was performed at the Krishna Shalby Hospital in Ahmedabad India, as part of Monogram’s multi-center clinical trial in India for the Monogram TKA System.

Figure 1: From left to right, Pankajkumar Yadav, Dr. Douglas Unis, Benjamin Sexson, Dr. Vikram Shah, Dr.Jawahar Pachore, Kamran Shamaei, Dr. Jayesh Patil, Aniruddh Nayak
As , Monogram and Shalby partnered to evaluate the safety and effectiveness of the mBôs TKA System with the Consensus CKS implant, which is substantially equivalent to the Monogram mPress implants for regulatory purposes. On April 29, 2025, Monogram obtained regulatory approval from India's Central Drugs Standard Control Organization ("CDSCO") to import its mBôs TKA system to conduct a multi-center clinical investigation evaluating the safety and effectiveness of the Monogram TKA System. The clinical trial includes 102 total knee replacement procedures, with a three-month clinical follow-up, conducted across multiple sites in India.
“The first fully autonomous knee replacement on a live patient using Monogram’s TKA System is a tremendous milestone for the Company. The procedure marks the culmination of years of hard work and dedication by our incredible team. I feel honored and proud to work with such incredible and dedicated individuals at Monogram. The system appears to have flawlessly executed the plan based on intraoperative surgeon evaluation, including intraoperative laxity estimation and achievement of planned alignment. I believe Monogram’s product will have a significant positive impact on the standard of care for orthopedic medicine in the years to come,�� said Benjamin Sexson, CEO of Monogram Technologies.
| �� |  |
“Watching the ease with which the system cut and executed the procedure was incredibly fulfilling. The safety and accuracy of the system were on obvious display. It really was an amazing moment watching Dr. Patil realize that robots can be more than marketing gimmicks but tools that actually change how knee replacements can be done better, said founder Dr. Douglas Unis. “Based on years of evaluating technology, watching this surgery was the final validation I needed to know with certainty that we have a groundbreaking product. It was incredible that there was so little stress, given the level of autonomy and first-time use on a living person. It was flawless.��
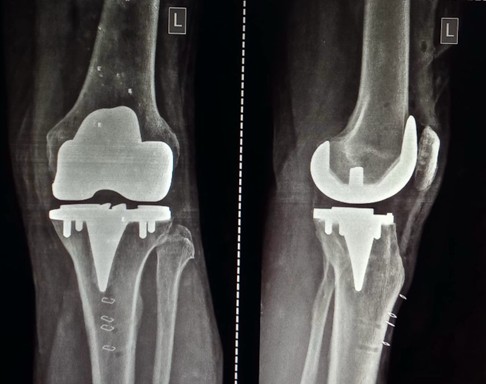
Figure 2: Post-operative x-ray. Intra-operative stressed laxity and limb alignment, and post-operative stressed laxities and limb alignment were assessed.
“Observing the flawless execution of this surgical plan was a highlight of my career and incredibly fulfilling. Patient safety has been painstakingly considered in virtually every aspect of our system. Our goal is to have the most efficient, most accurate and safest robot in the world. I am incredibly proud of the hard work of our team and to have had the opportunity to help build this technology,�� said Kamran Shamaei, Chief Technical Officer.
“I feel proud to have played a part in the development of the Monogram TKA System and to have been a member of this incredible team,�� said Ani Nayak, Sr. Manager of Clinical R&D. “As the individual responsible for the execution of this trial I feel a huge weight of responsibility. To see the system do its job with such low stress and execute the surgical plan as it did was extremely exciting. I believe our product will have a significant clinical impact globally.��
Strategic partner Shalby Limited (NSE: SHALBY) ("Shalby"), one of the world's largest orthopedic hospital groups, will support the 102-patient clinical investigation evaluating the safety and effectiveness of the Monogram TKA System, one of the world's first saw-based autonomous robots.
“I founded Shalby Hospitals to be a world-renowned center of excellence and innovation for joint replacement surgeries,�� said Dr. Vikram Shah, founder of Shalby Hospitals. “My vision has always been to pioneer advanced technologies in the field of orthopedic medicine. Having performed thousands of surgeries, I was deeply impressed by what the Monogram team has accomplished and by what was achieved in this surgery, and I look forward to supporting the introduction of this state-of-the-art robotic system, which reaffirms Shalby’s commitment to leading innovation and excellence in joint replacement surgeries.��
The U.S. Food and Drug Administration (FDA) for Monogram’s mBôs semi-autonomous TKA System.
Zimmer Biomet Holdings, Inc. (NYSE and SIX: ZBH), a global medical technology leader, and Monogram Technologies Inc. they entered into a definitive agreement for Zimmer Biomet to acquire all outstanding shares of stock of Monogram.
About Monogram Technologies Inc.
Monogram Technologies (NASDAQ: MGRM) is an AI-driven robotics company focused on improving human health, with an initial focus on orthopedic surgery. The Company is developing a product solution architecture to enable patient-optimized orthopedic implants at scale by combining 3D printing, advanced machine vision, AI and next-generation robotics.
To learn more, visit��.
Forward-Looking Statements
This press release may contain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Statements other than statements of historical facts included in this press release may constitute forward-looking statements and are not guarantees of future performance or results and involve a number of risks and uncertainties. Forward-looking statements, other than statements of historical fact, are highly likely to be affected by other unknowable future events and conditions, including elements of the future that are or are not under our control, and that the Company may or may not have considered; accordingly, such statements cannot be guarantees or assurances of any aspect of future performance. Actual developments and results are highly likely to vary materially from any forward-looking statements as a result of a number of factors, including those described in the Company's filings with the SEC. The Company undertakes no duty to update any forward-looking statement made herein. All forward-looking statements speak only as of the date of this press release.
Investor Relations
Chris Tyson
Executive Vice President
MZ North America
Direct: 949-491-8235
Photos accompanying this announcement are available at:��








