Silexion Therapeutics Announces New Preclinical Data Showing Up to 97% Inhibition of Cancer Cell Growth, Including New Evidence Against New Previously Untested KRAS Mutation
Silexion Therapeutics (NASDAQ: SLXN) has announced breakthrough preclinical data for its RNA interference therapy SIL204, demonstrating exceptional cancer cell growth inhibition rates. The study showed up to 97% inhibition in pancreatic cancer cells and 90% in colorectal cancer cells, including first-time evidence against the KRAS Q61H mutation.
Key findings revealed dose-dependent inhibition of up to 94% in pancreatic cancer cells with KRAS G12D mutations, 97% inhibition in pancreatic cancer cells with KRAS Q61H mutations, and nearly 90% inhibition in colorectal cancer cells with KRAS G12D mutations. The company is preparing for a Phase 2/3 clinical trial in Q2 2026 to investigate SIL204 for KRAS-driven solid tumor cancers.
Silexion Therapeutics (NASDAQ: SLXN) ha annunciato dati preclinici rivoluzionari per la sua terapia a interferenza RNA SIL204, dimostrando tassi eccezionali di inibizione della crescita delle cellule tumorali. Lo studio ha evidenziato fino al 97% di inibizione nelle cellule del cancro al pancreas e al 90% nelle cellule del cancro colorettale, includendo per la prima volta prove contro la mutazione KRAS Q61H.
I risultati chiave hanno mostrato un'inibizione dose-dipendente fino al 94% nelle cellule del cancro al pancreas con mutazioni KRAS G12D, un 97% di inibizione nelle cellule pancreatiche con mutazioni KRAS Q61H e quasi un 90% di inibizione nelle cellule colorettali con mutazioni KRAS G12D. L'azienda si sta preparando per una fase 2/3 di sperimentazione clinica nel secondo trimestre del 2026 per studiare SIL204 nei tumori solidi guidati da KRAS.
Silexion Therapeutics (NASDAQ: SLXN) ha anunciado datos preclÃnicos revolucionarios para su terapia de interferencia de ARN SIL204, demostrando tasas excepcionales de inhibición del crecimiento celular canceroso. El estudio mostró hasta un 97% de inhibición en células de cáncer pancreático y un 90% en células de cáncer colorrectal, incluyendo por primera vez evidencia contra la mutación KRAS Q61H.
Los hallazgos clave revelaron una inhibición dependiente de la dosis de hasta el 94% en células de cáncer pancreático con mutaciones KRAS G12D, un 97% de inhibición en células pancreáticas con mutaciones KRAS Q61H y casi un 90% de inhibición en células colorrectales con mutaciones KRAS G12D. La compañÃa se está preparando para un ensayo clÃnico de fase 2/3 en el segundo trimestre de 2026 para investigar SIL204 en cánceres sólidos impulsados por KRAS.
Silexion Therapeutics (NASDAQ: SLXN)ê° RNA ê°ì ì¹ë£ì � SIL204ì� ëí� í기ì ì¸ ì ìì� ë°ì´í°ë¥¼ ë°ííì¼ë©�, íìí� ìì¸í� ì±ì¥ ìµì ì¨ì ì ì¦íìµëë¤. ì°êµ¬ ê²°ê³¼ ì·ì¥ì� ì¸í¬ìì ìµë 97% ìµì , ëì¥ì ì¸í¬ìì 90% ìµì ë¥� ë³´ì¬ì£¼ìì¼ë©°, KRAS Q61H ë³ì´ì ëí� ìµì´ ì¦ê±°ë� í¬í¨ëììµëë�.
주ì ê²°ê³¼ë� KRAS G12D ë³ì´ê° ìë ì·ì¥ì� ì¸í¬ìì ì©ë ìì¡´ì � ìµì ìµë 94%, KRAS Q61H ë³ì´ê° ìë ì·ì¥ì� ì¸í¬ìì 97% ìµì , KRAS G12D ë³ì´ê° ìë ëì¥ì ì¸í¬ìì ê±°ì 90% ìµì ë¥� ëíëìµëë¤. íì¬ë� SIL204ë¥� KRAS 주ë ê³ í ì¢ ì ìì ëí� ì¡°ì¬í기 ìí 2026ë � 2ë¶ê¸° 2/3ì� ìììí ì¤ë¹� ì¤ì ëë¤.
Silexion Therapeutics (NASDAQ : SLXN) a annoncé des données précliniques révolutionnaires pour sa thérapie par interférence ARN SIL204, démontrant des taux exceptionnels d'inhibition de la croissance des cellules cancéreuses. L'étude a montré jusqu'à 97 % d'inhibition dans les cellules du cancer du pancréas et 90 % dans les cellules du cancer colorectal, incluant pour la première fois des preuves contre la mutation KRAS Q61H.
Les résultats clés ont révélé une inhibition dépendante de la dose allant jusqu'à 94 % dans les cellules du cancer du pancréas avec des mutations KRAS G12D, une inhibition de 97 % dans les cellules pancréatiques avec mutations KRAS Q61H, et près de 90 % d'inhibition dans les cellules colorectales avec mutations KRAS G12D. La société se prépare à un essai clinique de phase 2/3 au deuxième trimestre 2026 pour étudier SIL204 dans les cancers solides à mutation KRAS.
Silexion Therapeutics (NASDAQ: SLXN) hat bahnbrechende präklinische Daten für seine RNA-Interferenz-Therapie SIL204 veröffentlicht, die auÃergewöhnliche Hemmungsraten des Krebszellwachstums zeigen. Die Studie zeigte bis zu 97% Hemmung bei Bauchspeicheldrüsenkrebszellen und 90% bei kolorektalen Krebszellen, einschlieÃlich erstmals Nachweisen gegen die KRAS Q61H-Mutation.
Wichtige Ergebnisse zeigten eine dosisabhängige Hemmung von bis zu 94% bei Bauchspeicheldrüsenkrebszellen mit KRAS G12D-Mutationen, 97% Hemmung bei Bauchspeicheldrüsenkrebszellen mit KRAS Q61H-Mutationen und fast 90% Hemmung bei kolorektalen Krebszellen mit KRAS G12D-Mutationen. Das Unternehmen bereitet eine Phase-2/3-Studie im zweiten Quartal 2026 vor, um SIL204 bei KRAS-getriebenen soliden Tumoren zu untersuchen.
- Unprecedented inhibition rates of up to 97% in pancreatic cancer cells and 90% in colorectal cancer cells
- First evidence of efficacy against previously untested KRAS Q61H mutation
- Successful demonstration of broad applicability across three major cancer types - pancreatic, colorectal, and lung
- Validation of the company's innovative lipid-conjugated delivery system
- Phase 2/3 clinical trial not starting until Q2 2026, indicating a lengthy timeline to potential commercialization
Insights
Silexion's SIL204 shows remarkable 97% cancer cell inhibition across multiple KRAS mutations, significantly expanding its potential therapeutic applications.
Silexion's new preclinical data for their RNA interference therapy SIL204 represents a significant advancement in targeting KRAS-driven cancers. The unprecedented inhibition rates of up to 97% in pancreatic cancer cells and nearly 90% in colorectal cancer cells demonstrate exceptional potency at the preclinical stage.
Most notable is the newly demonstrated efficacy against the KRAS Q61H mutation, which hasn't previously been tested with SIL204. This substantially expands the potential application spectrum of the drug candidate as a pan-KRAS therapeutic. KRAS mutations drive some of the most aggressive and treatment-resistant cancers, and the Q61H variant represents an important additional target.
The dose-dependent response at nanomolar concentrations is particularly promising, suggesting potent activity at clinically achievable drug levels. The consistent effectiveness across pancreatic, colorectal, and lung cancer models â� the three most common KRAS-driven malignancies â� indicates broad therapeutic potential.
While these results are impressive, it's important to recognize they remain at the preclinical stage. The planned Phase 2/3 trial in Q2 2026 will be crucial to demonstrate whether this promising activity translates to clinical efficacy. The dual-route administration strategy (intratumoral and systemic delivery) could potentially address delivery challenges that have historically limited RNAi therapeutics.
If these inhibition rates translate to clinical settings, SIL204 could represent a significant advancement for patients with KRAS-driven cancers who currently have limited treatment options, particularly those with pancreatic cancer where effective therapies remain scarce.
New Results Reveal Unprecedented Inhibition Rates and First Evidence of SIL204's Efficacy Against Previously Untested KRAS Q61H Mutation in Human Cancer Cells, Significantly Expanding its Pan-KRAS Potential; Company on track for the initiation of a Phase 2/3 clinical trial in Q2 2026
Grand Cayman, July 31, 2025 (GLOBE NEWSWIRE) -- Silexion Therapeutics Corp. (NASDAQ: SLXN) ("Silexion" or the "Company"), a clinical-stage biotechnology company pioneering RNA interference (RNAi) therapies for KRAS-driven cancers, today announced groundbreaking new preclinical data revealing unprecedented inhibition rates of up to
While the Company has previously reported activity in various cancer models, these latest findings from a comprehensive CTG (Cell Titer-Glo) analysis demonstrate significantly higher inhibition rates and provide the first direct comparison of SIL204's potency across multiple cancer types and KRAS mutations in a single study, including dose-dependent inhibition of up to
Key findings from the preclinical studies include:
- SIL204 demonstrated dose-dependent inhibition of up to
94% in pancreatic cancer cells harboring KRAS G12D mutations at nanomolar concentrations - SIL204 showed comparable efficacy of approximately
97% inhibition in pancreatic cancer cells with KRAS Q61H mutations, a variant not previously reported in the Company's studies - SIL204 produced an inhibition rate of nearly
90% in colorectal cancer cells with KRAS G12D mutations, extending previous evidence of its effectiveness beyond pancreatic cancer
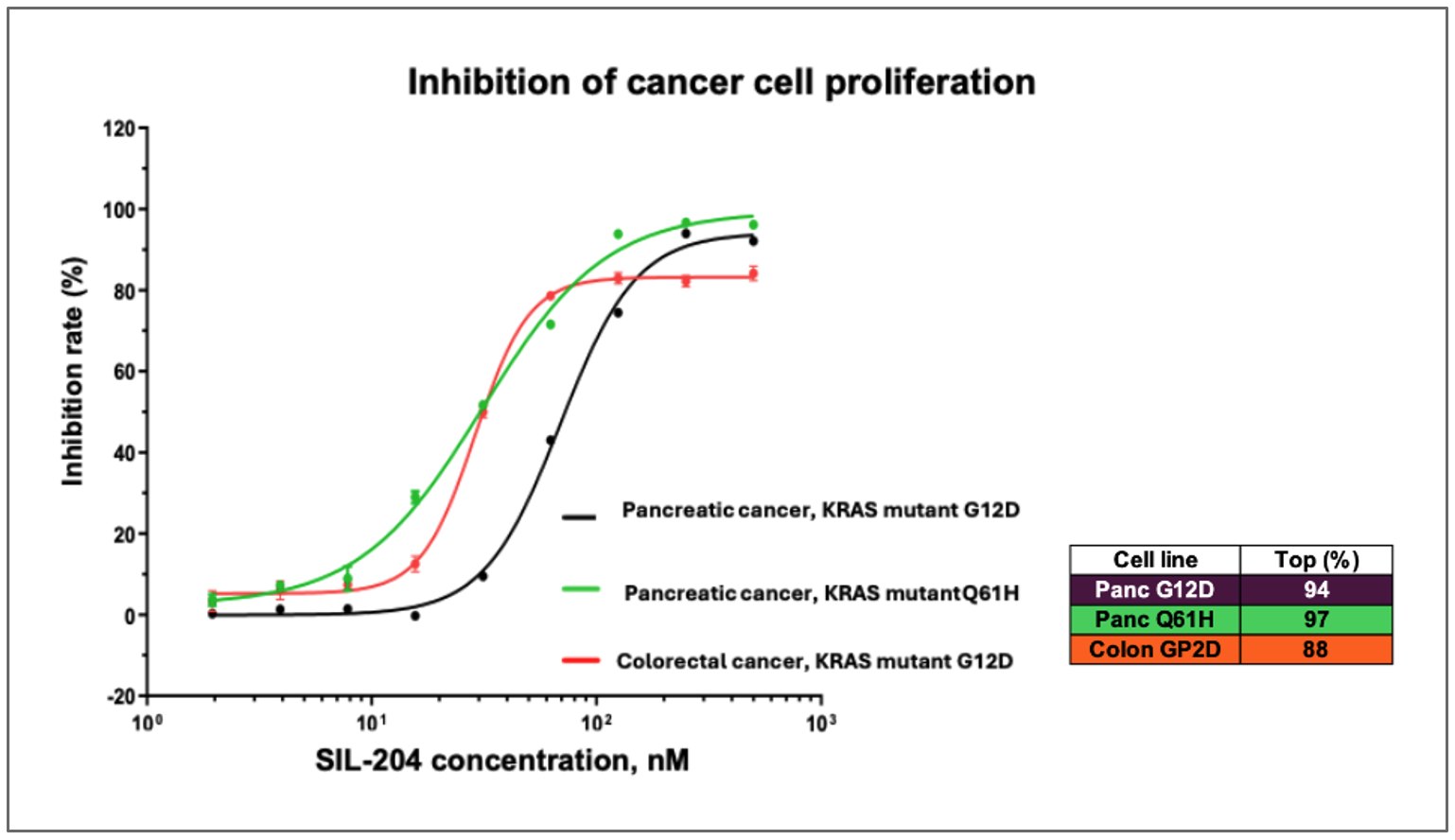
Figure: SIL204 demonstrates dose-dependent inhibition of cancer cell proliferation across  multiple tumor types and KRAS mutations, including pancreatic cancer with KRAS G12D (purple line) and Q61H mutations (turquoise line), as well as colorectal cancer with KRAS G12D mutation (orange line).
"These findings provide compelling evidence of SIL204's potent activity against multiple KRAS mutations across different cancer types," said Ilan Hadar, Chairman and Chief Executive Officer of Silexion. "The ability to achieve such high levels of inhibition in both pancreatic and colorectal cancer models with different KRAS mutations substantially strengthens SIL204's potential as a pan-KRAS therapeutic candidate. With these promising results across all three major KRAS-driven cancer types - pancreatic, colorectal, and lung - we're increasingly confident in SIL204's potential to address significant unmet needs for patients with these aggressive cancers."
This announcement comes just days after Silexion reported significant efficacy of SIL204 in lung cancer cell lines, validating the Company's innovative lipid-conjugated delivery system. Together, these results across pancreatic, colorectal, and lung cancer models provide comprehensive evidence of SIL204's broad applicability against KRAS-driven cancers.
"With our data now covering pancreatic, colorectal, and lung cancer models, we've demonstrated SIL204's consistent efficacy across the three most common KRAS-driven cancer types, added Ilan Hadar. Particularly notable is SIL204's high efficacy against the KRAS Q61H mutation, which expands our understanding of its potential against multiple KRAS variants."
Silexion continues to prepare for the initiation of a Phase 2/3 clinical trial in Q2 2026 to investigate SIL204 for the treatment of KRAS-driven solid tumor cancers, leveraging both intratumoral and systemic delivery approaches as part of the Company's dual-route administration strategy.
About Silexion Therapeutics
Silexion Therapeutics is a pioneering clinical-stage, oncology-focused biotechnology company developing innovative RNA interference (RNAi) therapies to treat solid tumors driven by KRAS mutations, the most common oncogenic driver in human cancers. The Companyâs first-generation product, LODERâ�, has shown promising results in a Phase 2 trial for non-resectable pancreatic cancer. Silexion is also advancing its next-generation siRNA candidate, SIL204, designed to target a broader range of KRAS mutations and showing significant potential in preclinical studies. The Company remains committed to pushing the boundaries of therapeutic innovation in oncology, with a focus on improving outcomes for patients with difficult-to-treat cancers. For more information please visit:Â
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the federal securities laws. All statements other than statements of historical fact contained in this communication, including statements regarding Silexion's business strategy, ongoing preclinical studies evaluating SIL204 in pancreatic and colorectal cancer applications, potential expansion of development strategy, and the therapeutic potential of SIL204 across multiple cancer types, are forward-looking statements. These forward-looking statements are generally identified by terminology such as "may", "should", "could", "might", "plan", "possible", "project", "strive", "budget", "forecast", "expect", "intend", "will", "estimate", "anticipate", "believe", "predict", "potential" or "continue", or the negatives of these terms or variations of them, or similar terminology. Forward-looking statements involve a number of risks, uncertainties, and assumptions, and actual results or events may differ materially from those projected or implied by those statements. Important factors that could cause such differences include, but are not limited to: (i) Silexion's ability to successfully complete preclinical studies and initiate clinical trials; (ii) Silexion's strategy, future operations, financial position, projected costs, prospects, and plans; (iii) the impact of the regulatory environment and compliance complexities; (iv) expectations regarding future partnerships or other relationships with third parties; (v) Silexion's future capital requirements and sources and uses of cash, including its ability to obtain additional capital; (vi) Silexionâs ability to maintain its Nasdaq listing; and (vii) other risks and uncertainties set forth in the documents filed or to be filed with the SEC by the Company, including the Company's Annual Report on Form 10-K for the year ended December 31, 2024, filed with the SEC on March 18, 2025. Silexion cautions you against placing undue reliance on forward-looking statements, which reflect current beliefs and are based on information currently available as of the date a forward-looking statement is made. Forward-looking statements set forth herein speak only as of the date they are made. Silexion undertakes no obligation to revise forward-looking statements to reflect future events, changes in circumstances, or changes in beliefs, except as otherwise required by law.
Company Contact
Silexion Therapeutics Corp
Ms. Mirit Horenshtein Hadar, CFO
Capital Markets & IR Contact
Arx Capital Markets
North American Equities Desk









