Vaxart Reports Additional Phase 1 Data Supporting the Potential Efficacy of its Second-Generation Norovirus Oral Pill Vaccine Candidate
Vaxart (OTCQX: VXRT) has reported promising Phase 1 data for its second-generation norovirus oral pill vaccine. The vaccine demonstrated a 25-fold increase in GII.4 fecal IgA response and a 10-fold increase in GI.I fecal IgA response over baseline with high-dose administration.
The second-generation constructs showed superior immunologic responses compared to first-generation versions, which previously demonstrated a 30% relative reduction in infection versus placebo in Phase 2b trials. The company's vaccine technology uniquely induces intestinal immunity, potentially offering stronger protection at the virus entry site.
With norovirus causing 685 million infections globally annually and representing a $10+ billion economic impact in the U.S., Vaxart is seeking partnerships to advance to Phase 2 trials by late 2025, with potential Phase 3 trials beginning in 2026.
Vaxart (OTCQX: VXRT) ha comunicato dati promettenti di Fase 1 per la sua seconda generazione di vaccino orale in compressa contro il norovirus. Il vaccino ha mostrato un aumento di 25 volte della risposta IgA fecale contro GII.4 e un aumento di 10 volte della risposta IgA fecale contro GI.1 rispetto ai livelli basali con somministrazione ad alto dosaggio.
Le formulazioni di seconda generazione hanno prodotto risposte immunologiche superiori rispetto alle versioni di prima generazione, che in precedenza avevano evidenziato una riduzione relativa del 30% delle infezioni rispetto al placebo negli studi di Fase 2b. La tecnologia vaccinale dell'azienda induce in modo unico l'immunità intestinale, offrendo potenzialmente una protezione più forte nel punto di ingresso del virus.
Poiché il norovirus causa 685 milioni di infezioni a livello globale ogni anno e rappresenta un impatto economico di oltre $10 miliardi negli USA, Vaxart cerca partnership per avanzare alla Fase 2 entro la fine del 2025, con potenziali studi di Fase 3 a partire dal 2026.
Vaxart (OTCQX: VXRT) ha informado datos prometedores de Fase 1 de su vacuna oral en pastilla de segunda generación contra el norovirus. La vacuna mostró un aumento de 25 veces en la respuesta de IgA fecal frente a GII.4 y un aumento de 10 veces en la respuesta de IgA fecal frente a GI.1 sobre la línea de base con la administración de dosis alta.
Las formulaciones de segunda generación presentaron respuestas inmunológicas superiores a las de primera generación, las cuales previamente demostraron una reducción relativa del 30% en las infecciones frente al placebo en ensayos de Fase 2b. La tecnología vacunal de la compañía induce de forma única inmunidad intestinal, lo que podría ofrecer una protección más fuerte en el punto de entrada del virus.
Dado que el norovirus provoca 685 millones de infecciones a nivel mundial al año y supone un impacto económico de más de $10 mil millones en EE. UU., Vaxart busca asociaciones para avanzar a la Fase 2 hacia finales de 2025, con posibles ensayos de Fase 3 a partir de 2026.
Vaxart (OTCQX: VXRT)�� 2세대 경구 정제�� 노로바이러스 백신�� 대�� 유망�� 1�� 데이터를 발표했습니다. 고용�� 투여 �� �� 백신은 기준�� 대�� GII.4�� 대�� 분변 IgA 반응�� 25�� 증가, GI.1�� 대�� 분변 IgA 반응�� 10�� 증가싵Ӽ�습니��.
2세대 구성체는 이전�� 1세대보다 우수�� 면역학적 반응�� 보였으며, 1세대�� 이전 2b�� 시험에서 플라시보 대�� 감염�� 상대적으�� 30% 감소�� 것으�� 나타났습니다. 회사�� 백신 기술은 ��(��) 면역�� 독특하게 유도�� 바이러스 침입 지점에�� �� 강한 보호�� 제공�� 가능성�� 있습니다.
노로바이러스�� �� 세계적으�� 매년 6��8500�� 건의 감염�� 일으키며 미국에서 100�� 달러 이상�� 경제�� 영향�� 초래하므��, Vaxart�� 2025�� 말까지 2�� 진입�� 위해 파트너십�� 모색하고 있으�� 2026년부�� 잠재�� 3�� 시험�� 시작�� 계획입니��.
Vaxart (OTCQX: VXRT) a publié des données de Phase 1 prometteuses pour son vaccin oral en comprimé de deuxième génération contre le norovirus. Le vaccin a montré une augmentation par 25 de la réponse IgA fécale contre GII.4 et une augmentation par 10 de la réponse IgA fécale contre GI.1 par rapport au niveau de base avec une administration à dose élevée.
Les constructions de deuxième génération ont entraîné des réponses immunologiques supérieures à celles de la première génération, qui avait précédemment montré une réduction relative de 30% des infections par rapport au placebo dans les essais de Phase 2b. La technologie vaccinale de la société induit de manière unique une immunité intestinale, offrant potentiellement une protection renforcée au point d'entrée du virus.
Étant donné que le norovirus provoque 685 millions d'infections dans le monde chaque année et représente un impact économique de plus de 10 milliards de dollars aux États‑Unis, Vaxart recherche des partenariats pour passer en Phase 2 d'ici fin 2025, avec d'éventuels essais de Phase 3 débutant en 2026.
Vaxart (OTCQX: VXRT) hat vielversprechende Phase��1‑Daten für seinen oralen Tablettenimpfstoff der zweiten Generation gegen Norovirus berichtet. Der Impfstoff zeigte bei hoher Dosis eine 25‑fache Steigerung der fäkalen IgA‑Antwort gegen GII.4 und eine 10‑fache Steigerung der fäkalen IgA‑Antwort gegen GI.1 gegenüber dem Ausgangswert.
Die Konstrukte der zweiten Generation führten zu überlegenen immunologischen Reaktionen im Vergleich zur ersten Generation, die zuvor in Phase��2b‑Studien eine relative Reduktion der Infektionen um 30% gegenüber Placebo zeigte. Die Impfstofftechnik des Unternehmens induziert einzigartig eine intestinale Immunität und könnte so einen stärkeren Schutz an der Eintrittsstelle des Virus bieten.
Da Norovirus jährlich 685 Millionen Infektionen weltweit verursacht und in den USA einen wirtschaftlichen Schaden von über 10 Milliarden US‑Dollar anrichtet, sucht Vaxart Partnerschaften, um bis Ende 2025 in Phase 2 vorzurücken, mit möglichen Phase��3‑Studien ab 2026.
- None.
- Company requires partnership or additional funding to proceed with Phase 2 trials
- Phase 1 study was not powered to determine statistical superiority
Insights
Vaxart's second-gen norovirus vaccine shows superior immunological responses, suggesting better efficacy potential than its first-gen predecessor that already demonstrated 30% protection.
The Phase 1 data for Vaxart's second-generation norovirus oral pill vaccine shows significant immunological improvements over their first-generation candidate. The new constructs demonstrated a 25-fold increase in GII.4 fecal IgA and a 10-fold increase in GI.1 fecal IgA responses with high-dose administration. Even at low doses, robust responses were observed (8-fold for GII.4 and 7-fold for GI.1).
These improvements are particularly meaningful because Vaxart's previous Phase 2b challenge study identified fecal IgA as a critical correlate of protection against norovirus infection. The first-generation constructs had already shown a statistically significant 30% relative reduction in infection compared to placebo, suggesting this enhanced immune response could translate to improved efficacy.
The data also reinforces Vaxart's differentiated approach focusing on intestinal immunity rather than just serum antibodies. Earlier results from June 2025 showed that these second-generation constructs produced statistically significant increases in norovirus blocking antibodies (141% for GI.1 and 94% for GII.4) compared to first-generation constructs.
While promising, several hurdles remain before commercialization. The company is actively seeking partnerships or funding for a planned Phase 2b safety and immunogenicity study, potentially beginning before year-end 2025, with Phase 3 possibly starting in 2026. The norovirus market opportunity is substantial, with approximately 685 million global infections annually (20 million in the US) and an estimated economic burden of $60 billion worldwide ($10 billion in the US).
- Second-generation constructs induce robust increases in fecal IgA, which was shown to be correlated with protection against infection in the company’s previous Phase 2b challenge study -
SOUTH SAN FRANCISCO, Calif., Sept. 10, 2025 (GLOBE NEWSWIRE) -- Vaxart, Inc. (OTCQX: VXRT), a clinical-stage biotechnology company developing a range of oral recombinant pill vaccines based on its proprietary delivery platform, today reported additional positive data from the Phase 1 clinical trial evaluating its second-generation oral pill norovirus vaccine constructs head-to-head against its first-generation constructs. Dr. Sean Tucker, Vaxart’s Founder and Chief Scientific Officer, presented the data at the 9th International Calicivirus Conference, which is taking place in Banff, Canada, September 7-11, 2025. Further, Dr. Becca Flitter presented data on Vaxart’s norovirus challenge study at the same conference.
The data show a 25-fold increase in the GII.4 fecal IgA response and a 10-fold increase in the GI.I fecal IgA response over baseline with the high dose of the second-generation vaccine candidates after a single tablet administration for each strain. The data also show an 8-fold increase in the GII.4 fecal IgA response and a 7-fold increase in the GI.I fecal IgA response over baseline with the low dose of the second-generation vaccine candidates after a single tablet administration for each strain.
While the Phase 1 study was not powered to determine superiority by statistical methods, the fecal IgA increases observed with the second-generation constructs compared favorably to the increases observed with the first-generation constructs at the same high dose level and in the same study (13-fold GII.4 and 6-fold GI.1 over baseline). As , a Phase 2 challenge study of the first-generation constructs identified fecal IgA as a critical correlate to protection from norovirus infection.1
“With these positive fecal IgA results and the previously announced serum responses, the totality of data has given us confidence that our second-generation constructs induce more robust immunologic responses than our first-generation constructs. These immunological endpoints are important because they correlated with protection in our Phase 2b challenge study,�� said Dr. Tucker. “As the first-generation constructs demonstrated a statistically significant
These new data confirm that Vaxart’s vaccine technology, which induces intestinal immunity, likely enables stronger protection against infection from norovirus by providing better immunity at the site of virus entry. With second-generation constructs that produces more intestinal IgA, these data further differentiate Vaxart’s norovirus vaccine approach from others in development, which are more focused on generating a serum antibody response.
In June 2025, Vaxart from the Phase 1 head-to-head study demonstrating that its second-generation norovirus constructs produced statistically significant increases in GI.1 and GII.4 norovirus blocking antibodies (
“Results to date on all our norovirus vaccines studies give us confidence that we may have a potential solution for an easily transmitted disease with a
Assuming a partnership or other funding, Vaxart plans to conduct a Phase 2b safety and immunogenicity study that could potentially begin before the end of 2025 followed by an End of Phase 2 meeting with the U.S. Food and Drug Administration (FDA). A Phase 3 trial could then begin as early as 2026.
There is no approved vaccine against norovirus, a leading cause of acute gastroenteritis (AGE) worldwide that is responsible for outbreaks of infection and illness globally. Each year there are approximately 685 million norovirus infections globally, with 20 million infections occurring annually in the United States. Due to these high rates of infection, norovirus is believed to cause nearly
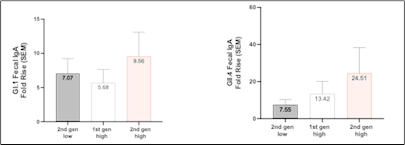
Fecal IgA response data from Vaxart’s Phase 1 norovirus study
Reference
1 Flitter BA, Gillard J, Greco SN et al. An oral norovirus vaccine generates mucosal immunity and reduces viral shedding in a phase 2 placebo-controlled challenge study. 2025;17(798):eadh9906.
�����dzܳ����ղ��油������
Vaxart is a clinical-stage biotechnology company developing a range of oral recombinant vaccines based on its proprietary delivery platform. Vaxart vaccines are designed to be administered using pills that can be stored and shipped without refrigeration and eliminate the risk of needle-stick injury. Vaxart believes that its proprietary pill vaccine delivery platform is suitable to deliver recombinant vaccines, positioning the company to develop oral versions of currently marketed vaccines and to design recombinant vaccines for new indications. Vaxart’s development programs currently include pill vaccines designed to protect against coronavirus, norovirus and influenza, as well as a therapeutic vaccine for human papillomavirus (HPV), Vaxart’s first immune-oncology indication. Vaxart has filed broad domestic and international patent applications covering its proprietary technology and creations for oral vaccination using adenovirus and TLR3 agonists.
Note Regarding Forward-Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, included in this press release regarding Vaxart's strategy, prospects, plans and objectives, funding milestones, the results of the FDA’s review of any trials, studies, or data, results from clinical trials and the timing of such results and such trials, commercialization agreements and licenses, and beliefs and expectations of management are forward-looking statements. These forward-looking statements may be accompanied by such words as “should,�� “believe,�� “could,�� “potential,�� “will,�� “expected,�� “anticipate,�� “plan,�� and other words and terms of similar meaning. Examples of such statements include, but are not limited to, statements relating to Vaxart’s ability to complete the Phase 1 trial of its oral bivalent norovirus vaccine; Vaxart's ability to develop and commercialize its product candidates, including its vaccine booster products; Vaxart's expectations regarding clinical results and trial data, including their design, and the timing of such trials and of receiving and reporting such clinical results and trial data; Vaxart’s expectations regarding timing of enrollment in studies; and Vaxart's expectations with respect to the effectiveness of its product candidates and the potential of its vaccine pill platform. Vaxart may not actually achieve the plans, carry out the intentions, or meet the expectations or projections disclosed in the forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions, expectations, and projections disclosed in the forward-looking statements. Various important factors could cause actual results or events to differ materially from the forward-looking statements that Vaxart makes, including uncertainties inherent in research and development, including the ability to meet anticipated clinical endpoints, commencement, and/or completion dates for clinical trials, regulatory submission dates, regulatory approval dates, and/or launch dates, as well as the possibility of unfavorable new clinical data and further analyses of existing clinical data; the risk that clinical trial data are subject to differing interpretations and assessments by regulatory authorities; whether regulatory authorities will be satisfied with the design of and results from the clinical studies; decisions by regulatory authorities impacting labeling, manufacturing processes, and safety that could affect the availability or commercial potential of any product candidate, including the possibility that Vaxart's product candidates may not be approved by the FDA or non-U.S. regulatory authorities; that, even if approved by the FDA or non-U.S. regulatory authorities, Vaxart's product candidates may not achieve broad market acceptance; that a Vaxart collaborator may not attain development and commercial milestones; that Vaxart or its partners may experience manufacturing issues and delays due to events within, or outside of, Vaxart's or its partners' control; difficulties in production, particularly in scaling up initial production, including difficulties with production costs and yields, quality control, including stability of the product candidate and quality assurance testing, shortages of qualified personnel or key raw materials, and compliance with strictly enforced federal, state, and foreign regulations; that Vaxart may not be able to obtain, maintain, and enforce necessary patent and other intellectual property protection; that Vaxart's capital resources may be inadequate; Vaxart's ability to resolve pending legal matters; Vaxart's ability to obtain sufficient capital to fund its operations on terms acceptable to Vaxart, if at all; the impact of government healthcare proposals and policies; competitive factors; and other risks described in the "Risk Factors" sections of Vaxart's Quarterly and Annual Reports filed with the U.S. Securities and Exchange Commission. Vaxart does not assume any obligation to update any forward-looking statements, except as required by law.
Contact
Vaxart Media and Investor Relations:
Matt Steinberg
FINN Partners
(646) 871-8481
A photo accompanying this announcement is available at
This press release was published by a CLEAR® Verified individual.










