BriaCell Patient Achieves Sustained Complete Resolution of Lung Metastasis in Bria-OTSâ� Metastatic Breast Cancer Study
BriaCell Therapeutics (NASDAQ: BCTX) reported sustained complete resolution of lung metastasis in the first patient treated with Bria-OTS, their personalized off-the-shelf immunotherapy for metastatic breast cancer. The 78-year-old patient with hormone receptor-positive, HER2-negative metastatic breast cancer showed 100% resolution of lung metastasis, maintained at 2, 4, and 6 months after treatment.
The patient, who had multiple prior treatment failures, has received 12 cycles of Bria-OTS monotherapy with no treatment-limiting toxicities. This milestone was achieved at the lowest dose level, demonstrating strong single-agent activity in a challenging patient population.
BriaCell Therapeutics (NASDAQ: BCTX) ha riportato una completa risoluzione duratura delle metastasi polmonari nel primo paziente trattato con Bria-OTS, la loro immunoterapia personalizzata pronta all'uso per il carcinoma mammario metastatico. Il paziente di 78 anni con carcinoma mammario metastatico positivo ai recettori ormonali e HER2-negativo ha mostrato una risoluzione del 100% delle metastasi polmonari, mantenuta a 2, 4 e 6 mesi dal trattamento.
Il paziente, che aveva subito diversi fallimenti terapeutici precedenti, ha ricevuto 12 cicli di monoterapia con Bria-OTS senza tossicità che limitassero il trattamento. Questo traguardo è stato raggiunto al livello di dose più basso, dimostrando una forte attività del singolo agente in una popolazione di pazienti difficile.
BriaCell Therapeutics (NASDAQ: BCTX) informó una resolución completa y sostenida de las metástasis pulmonares en el primer paciente tratado con Bria-OTS, su inmunoterapia personalizada lista para usar para cáncer de mama metastásico. El paciente de 78 años con cáncer de mama metastásico positivo a receptores hormonales y HER2-negativo mostró una resolución del 100% de las metástasis pulmonares, mantenida a los 2, 4 y 6 meses después del tratamiento.
El paciente, que habÃa tenido múltiples fracasos en tratamientos previos, recibió 12 ciclos de monoterapia con Bria-OTS sin toxicidades que limitaran el tratamiento. Este logro se consiguió con la dosis más baja, demostrando una fuerte actividad del agente único en una población de pacientes difÃcil.
BriaCell Therapeutics (NASDAQ: BCTX)ë� ì ì´ì� ì ë°©ìì ìí ê°ì¸ ë§ì¶¤í� ì¦ì ì¬ì© ê°ë¥í ë©´ìì¹ë£ì � Bria-OTSë¡� ì¹ë£ë°ì ì²� ë²ì§¸ íììì í� ì ì´ì� ìì í� ì§ìì ìì¤ì� ë³´ê³ íìµëë¤. 78ì� íìë� í¸ë¥´ëª� ìì©ì²� ìì±, HER2 ìì± ì ì´ì� ì ë°©ìì ìê³ ììì¼ë©°, ì¹ë£ í� 2, 4, 6ê°ìì� ê±¸ì³ í� ì ì´ê° 100% ìì¤ë� ìíë¥� ì ì§íìµëë¤.
ì¬ë¬ ì°¨ë¡ì� ì´ì ì¹ë£ ì¤í¨ë¥� 겪ì ì� íìë� 12주기ì� Bria-OTS ë¨ë ìë²ì� ë°ìì¼ë©° ì¹ë£ë¥� ì ííë ë ì±ì ìììµëë�. ì� ì´ì íë ê°ì� ë®ì ì©ë ìì¤ìì ë¬ì±ëì´, ì´ë ¤ì� íìêµ°ìì� ë¨ì¼ ì½ì ì� ê°ë ¥í� í¨ë¥ì� ì ì¦íìµëë¤.
BriaCell Therapeutics (NASDAQ : BCTX) a rapporté une résolution complète et durable des métastases pulmonaires chez le premier patient traité avec Bria-OTS, leur immunothérapie personnalisée prête à l'emploi pour le cancer du sein métastatique. Le patient de 78 ans atteint d'un cancer du sein métastatique hormonosensible et HER2-négatif a montré une résolution à 100 % des métastases pulmonaires, maintenue à 2, 4 et 6 mois après le traitement.
Le patient, qui avait connu plusieurs échecs de traitements antérieurs, a reçu 12 cycles de monothérapie Bria-OTS sans toxicités limitant le traitement. Cette étape a été atteinte avec la dose la plus faible, démontrant une forte activité du traitement en monothérapie dans une population de patients difficile.
BriaCell Therapeutics (NASDAQ: BCTX) berichtete über eine anhaltende vollständige Rückbildung der Lungenmetastasen beim ersten Patienten, der mit Bria-OTS, ihrer personalisierten, sofort einsatzbereiten Immuntherapie für metastasierten Brustkrebs, behandelt wurde. Der 78-jährige Patient mit hormonrezeptor-positivem, HER2-negativem metastasiertem Brustkrebs zeigte eine 100%ige Rückbildung der Lungenmetastasen, die 2, 4 und 6 Monate nach der Behandlung anhielt.
Der Patient, der zuvor mehrere Therapieversagen erlitten hatte, erhielt 12 Zyklen Bria-OTS-Monotherapie ohne behandlungsbegrenzende Toxizitäten. Dieser Meilenstein wurde bei der niedrigsten Dosisstufe erreicht und zeigt eine starke Einzelwirkstoffaktivität in einer herausfordernden Patientengruppe.
- Complete (100%) resolution of lung metastasis maintained for 6 months
- No treatment-limiting toxicities observed in the patient
- Strong single agent activity achieved at lowest dose level
- Patient remains on study with stable disease
- Results limited to only one patient so far
- Study still in early Phase 1/2a stage
Insights
BriaCell's Bria-OTS therapy shows remarkable durability with complete resolution of lung metastasis maintained at 6 months in first patient.
The clinical data presented in this announcement represents a potentially significant development in immunotherapy for metastatic breast cancer. A 78-year-old patient with hormone receptor-positive, HER2-negative metastatic breast cancer who had experienced multiple prior treatment failures has shown a complete response in lung metastasis following only four doses of Bria-OTS monotherapy. Most importantly, this response has been maintained for 6 months, suggesting meaningful durability.
From a clinical perspective, several elements make this result particularly noteworthy. First, this response was achieved with monotherapy rather than a combination approach, which is uncommon in advanced metastatic disease. Second, the patient population â� elderly with multiple treatment failures â� represents a challenging cohort with typically poor prognosis. Third, the response occurred at the lowest dose level of the Phase 1/2a study, suggesting potential for even greater efficacy at higher doses.
The lack of treatment-limiting toxicities is equally important in this elderly patient population, where tolerability often determines whether a therapy can be continued. The patient has now received 12 treatment cycles, indicating acceptable long-term tolerability.
While this represents data from just a single patient and broader efficacy patterns remain to be established, complete responses with durability beyond 6 months in heavily pretreated metastatic breast cancer patients are relatively rare. The company's plans to evaluate Bria-OTS in combination with checkpoint inhibitors represents a logical next step, as such combinations often show synergistic effects in immunotherapy approaches.
- [IMAGES BELOW] Complete resolution maintained at 6 months in first patient treated with BriaCellâs Bria-OTS in Phase 1/2a study
- No treatment limited toxicities observed
- Patient remains on study with stable disease elsewhere
PHILADELPHIA and VANCOUVER, British Columbia, July 09, 2025 (GLOBE NEWSWIRE) -- BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW, BCTXZ) (TSX: BCT) (âBriaCellâ� or the âCompanyâ�), a clinical-stage biotechnology company developing novel immunotherapies to transform cancer care, today announced the sustained complete resolution of lung metastasis in a patient with hormone receptor-positive (HR+), HER2-negative, metastatic breast cancer (MBC) treated with Bria-OTS, the Companyâs personalized off the shelf immunotherapy.
BriaCellâs first Bria-OTS study patient, a 78-year-old woman with advanced disease and multiple prior treatment failures, achieved
Figure 1: Treatment with Bria-OTS monotherapy resulted in
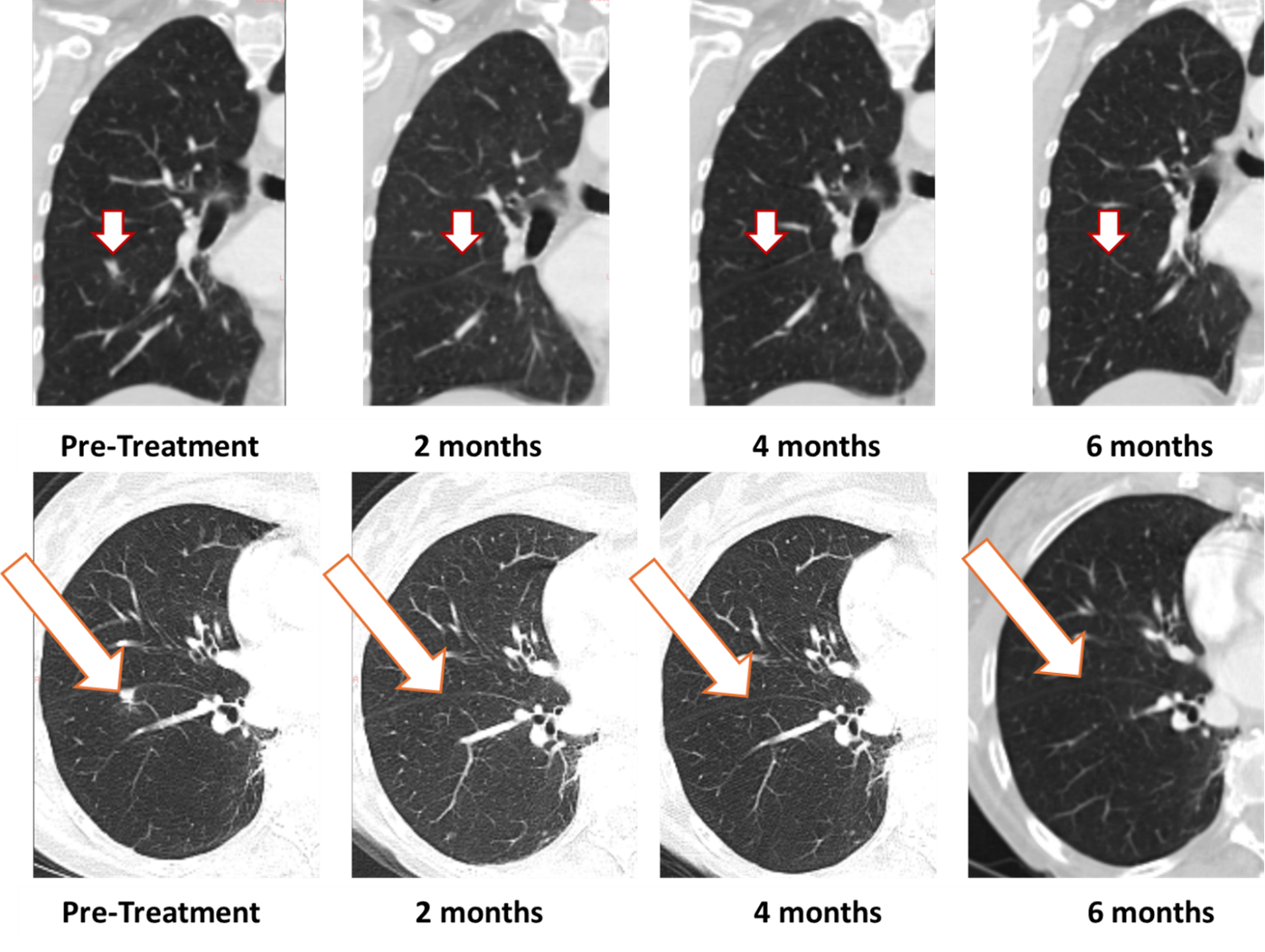
âThese results represent an exciting clinical milestone in the Bria-OTS program,â� stated Neal S. Chawla MD, Director at the Sarcoma Oncology Center, Santa Monica, CA, and Principal Investigator for the Bria-OTS study. âWe are seeing strong single agent activity in a very challenging population and are eager to explore this approach across more patient subtypes and tumors.â�
âWe are highly encouraged by this remarkable and durable clinical response, especially at the lowest dose level,â� added Dr. William V. Williams, BriaCellâs President and CEO. âThis data underscores the therapeutic potential of our Bria-OTS platform, and we look forward to further evaluating it in combination with a checkpoint inhibitor to improve outcomes in patients with advanced breast cancer.â�
About Bria-OTS
Bria-OTS is a next generation, off-the-shelf personalized immunotherapy based on BriaCellâs lead candidate Bria-IMT currently being evaluated in a Phase 1/2a study (ClinicalTrials.gov identifier: ) in patients with metastatic recurrent breast cancer. The trial includes both monotherapy dose escalation and check point inhibition combination dose expansion cohorts. The Company recently progressed into the dose expansion phase.
About BriaCell Therapeutics Corp.
BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at .
Safe Harbor
This press release contains âforward-looking statementsâ� that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release include statements regarding: BriaCell continuing the Phase 1/2a Bria-OTS study and reproducing similar results in patients with MBC and other cancers; the use of the Bria-OTS platform as monotherapy; and Bria-OTSâs validation as a personalized immunotherapy approach. Forward-looking statements may be identified by the use of words such as âanticipate,â� âbelieve,â� âcontemplate,â� âcould,â� âestimate,â� âexpect,â� âintend,â� âseek,â� âmay,â� âmight,â� âplan,â� âpotential,â� âpredict,â� âproject,â� âtarget,â� âaim,â� âshould,â� âwill,â� âwould,â� or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading âRisks and Uncertaintiesâ� in the Companyâs most recent Managementâs Discussion and Analysis, under the heading âRisk Factorsâ� in the Companyâs most recent Annual Information Form, and under âRisks and Uncertaintiesâ� in the Companyâs other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Companyâs profiles on SEDAR+ atÌýÌýand on EDGAR atÌý. Forward-looking statements contained in this announcement are made as of this date, and BriaCell Therapeutics Corp. undertakes no duty to update such information except as required under applicable law.
Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release.
Contact Information
Company Contact:
William V. Williams, MD
President & CEO
1-888-485-6340
Ìý
Investor Relations Contact:
1 Note that the other white dots in the lungs are blood vessels.
A photo accompanying this announcement is available at .








