Vivos Therapeutics Receives Medicare Approval for VidaSleepā� Oral Appliance
Vivos Therapeutics (NASDAQ: VVOS) has achieved a significant milestone as its VidaSleepā� oral appliance received approval from the Centers for Medicare & Medicaid Services (CMS) for treating mild to moderate obstructive sleep apnea (OSA) and snoring in adults.
The VidaSleepā� device, featuring Vivos' patented and FDA-cleared Unilateral Bite Block technology, joins the company's mmRNAĀ® device on the PDAC list of covered oral appliances. This dual-PDAC approval makes Vivos the only company with two Medicare-covered oral appliances, expanding access to millions of Medicare beneficiaries.
With an estimated 80 million Americans affected by OSA and over 80% of cases undiagnosed, Vivos is targeting the $36 billion sleep therapy market. The VidaSleepā� device is designed to offer a cost-effective solution without compromising efficacy, maintaining strong gross margins while expanding accessibility through Medicare and commercial insurance networks.
Vivos Therapeutics (NASDAQ: VVOS) ha raggiunto un traguardo importante: il suo dispositivo orale VidaSleepā� ha ottenuto l'approvazione dai Centers for Medicare & Medicaid Services (CMS) per il trattamento dell'apnea ostruttiva del sonno (OSA) da lieve a moderata e del russamento negli adulti.
Il dispositivo VidaSleepā�, che incorpora la tecnologia Unilateral Bite Block brevettata da Vivos e approvata dalla FDA, si aggiunge al dispositivo mmRNAĀ® dell'azienda nella lista PDAC degli apparecchi orali coperti. Questa doppia approvazione PDAC rende Vivos l'unica azienda con due dispositivi orali coperti da Medicare, ampliando cosƬ l'accesso a milioni di beneficiari Medicare.
Con circa 80 milioni di americani affetti da OSA e oltre l'80% dei casi non diagnosticati, Vivos punta al mercato della terapia del sonno da 36 miliardi di dollari. Il dispositivo VidaSleepā� ĆØ progettato per offrire una soluzione efficiente in termini di costi senza compromettere l'efficacia, mantenendo margini lordi elevati e ampliando l'accessibilitĆ tramite Medicare e reti assicurative commerciali.
Vivos Therapeutics (NASDAQ: VVOS) ha alcanzado un hito importante al obtener la aprobaciĆ³n de los Centers for Medicare & Medicaid Services (CMS) para su dispositivo oral VidaSleepā�, destinado al tratamiento de la apnea obstructiva del sueƱo (AOS) leve a moderada y el ronquido en adultos.
El dispositivo VidaSleepā�, que cuenta con la tecnologĆa Unilateral Bite Block patentada y aprobada por la FDA de Vivos, se suma al dispositivo mmRNAĀ® de la compaƱĆa en la lista PDAC de dispositivos orales cubiertos. Esta doble aprobaciĆ³n PDAC convierte a Vivos en la Ćŗnica empresa con dos dispositivos orales cubiertos por Medicare, ampliando el acceso a millones de beneficiarios de Medicare.
Con aproximadamente 80 millones de estadounidenses afectados por AOS y mĆ”s del 80% de los casos sin diagnosticar, Vivos apunta al mercado de terapias para el sueƱo de 36 mil millones de dĆ³lares. El dispositivo VidaSleepā� estĆ” diseƱado para ofrecer una soluciĆ³n rentable sin sacrificar la eficacia, manteniendo mĆ”rgenes brutos sĆ³lidos y ampliando la accesibilidad a travĆ©s de Medicare y redes de seguros comerciales.
Vivos Therapeutics (NASDAQ: VVOS)µē� VidaSleepā� źµ¬ź° ģ„ģ¹ź° ź²½ģ¦ģģ ģ¤ė±ėģ ķģģ� ģė©“ ė¬“ķøķ”ģ¦(OSA) ė°� ģ±ģøģ� ģ½ź³Øģ� ģ¹ė£ė„� ģķ“ Medicare ė°� Medicaid ģė¹ģ� ģ¼ķ°(CMS)ģ� ģ¹ģøģ� ė°µē ģ¤ģķ� ģ“ģ ķė„¼ ė¬ģ±ķģµėė¤.
Vivosģ� ķ¹ķė°ģ FDA ģ¹ģø ėØģ¼ źµķ© ėøė” źø°ģ ģ� ģ ģ©ė� VidaSleepā� ģ„ģ¹µē� ķģ¬ģ� mmRNAĀ® ģ„ģ¹ģ ķØź» PDACģģ ė³“ķ ģ ģ© źµ¬ź° ģ„ģ¹ ėŖ©ė”ģ� ķ¬ķØėģģµėė�. ģ“ģ¤ PDAC ģ¹ģøģ� ķµķ“ Vivosµē� Medicare ė³“ķ ģ ģ© źµ¬ź° ģ„ģ¹ė„� ė� ź°� ė³“ģ ķ� ģ ģ¼ķ� ķģ¬ź° ėģ“ ģė°±ė§� Medicare ģķģģ ģ ź·¼ģ±ģ ķėķģµėė¤.
ģ� 8ģ²ė§ ėŖ ģ ėÆøźµģøģ“ OSAģ� ģķ„ģ� ė°ź³ 80% ģ“ģģ� ģ¬ė”µē� ģ§ėØėģ§ ģģ ģķģģ, Vivosµē� 360ģ� ė¬ė¬ ź·ėŖØģ� ģė©“ ģ¹ė£ ģģ„ģ� ź²Øė„ķź³ ģģµėė¤. VidaSleepā� ģ„ģ¹µē� ė¹ģ© ķØģØģ ģø ģė£Øģ ģ ģ ź³µķė©“ģė ķØė„ģ� ģ ģ§ķėė”� ģ¤ź³ėģ“, ź°ė „ķ� ģ“� ģ“ģµė„ ģ ģ ģ§ķź³ Medicare ė°� ģģ ė³“ķ ė¤ķøģķ¬ė„� ķµķ“ ģ ź·¼ģ±ģ ķėķ©ėė�.
Vivos Therapeutics (NASDAQ : VVOS) a franchi une Ć©tape importante avec l'approbation de son appareil buccal VidaSleepā� par les Centers for Medicare & Medicaid Services (CMS) pour le traitement de l'apnĆ©e obstructive du sommeil (AOS) lĆ©gĆØre Ć modĆ©rĆ©e et du ronflement chez les adultes.
L'appareil VidaSleepā�, intĆ©grant la technologie brevetĆ©e Unilateral Bite Block de Vivos, approuvĆ©e par la FDA, rejoint le dispositif mmRNAĀ® de la sociĆ©tĆ© sur la liste PDAC des appareils buccaux couverts par l'assurance. Cette double approbation PDAC fait de Vivos la seule entreprise Ć disposer de deux appareils buccaux pris en charge par Medicare, Ć©largissant ainsi l'accĆØs Ć des millions de bĆ©nĆ©ficiaires Medicare.
Avec environ 80 millions d'AmĆ©ricains touchĆ©s par l'AOS et plus de 80 % des cas non diagnostiquĆ©s, Vivos vise le marchĆ© des thĆ©rapies du sommeil de 36 milliards de dollars. L'appareil VidaSleepā� est conƧu pour offrir une solution rentable sans compromettre l'efficacitĆ©, en maintenant de fortes marges brutes tout en Ć©largissant l'accessibilitĆ© via Medicare et les rĆ©seaux d'assurance commerciaux.
Vivos Therapeutics (NASDAQ: VVOS) hat einen bedeutenden Meilenstein erreicht, da sein VidaSleepā� MundgerƤt von den Centers for Medicare & Medicaid Services (CMS) zur Behandlung von leichtem bis mittelschwerem obstruktivem Schlafapnoe-Syndrom (OSA) und Schnarchen bei Erwachsenen zugelassen wurde.
Das VidaSleepā� GerƤt, das Vivos' patentierte und von der FDA zugelassene Unilateral Bite Block-Technologie verwendet, ergƤnzt das mmRNAĀ® GerƤt des Unternehmens auf der PDAC-Liste der erstattungsfƤhigen MundgerƤte. Diese doppelte PDAC-Zulassung macht Vivos zum einzigen Unternehmen mit zwei Medicare-erstatteten MundgerƤten und erweitert den Zugang fĆ¼r Millionen Medicare-Berechtigte.
Mit geschƤtzten 80 Millionen Amerikanern, die von OSA betroffen sind, und Ć¼ber 80 % der FƤlle, die nicht diagnostiziert werden, zielt Vivos auf den 36 Milliarden Dollar groĆen Markt fĆ¼r Schlaftherapien ab. Das VidaSleepā� GerƤt ist darauf ausgelegt, eine kosteneffiziente Lƶsung ohne EinbuĆen bei der Wirksamkeit zu bieten, dabei starke Bruttomargen zu halten und die ZugƤnglichkeit durch Medicare und kommerzielle Versicherungsnetzwerke zu erweitern.
- Medicare approval enables access for millions of beneficiaries
- Only company with two Medicare-covered oral appliances
- Targets $36 billion sleep therapy market opportunity
- Maintains strong gross profit margins despite cost-effective pricing
- Approval likely to influence coverage by private insurers
- Device can be used as standalone therapy or with CPAP
- Enters a market with 80% of cases currently undiagnosed
- Faces competition from established CPAP machines and surgical options
Insights
Medicare approval of VidaSleep gives Vivos access to millions of beneficiaries, significantly expanding market reach for its OSA treatment.
Vivos Therapeutics has achieved a significant regulatory milestone with Medicare's approval of its VidaSleepā� oral appliance for treating mild to moderate obstructive sleep apnea (OSA). This approval from the Centers for Medicare & Medicaid Services (CMS) Pricing, Data Analysis and Coding contractor represents a major market expansion opportunity.
The strategic importance cannot be overstatedāthis is Vivos' second device to receive PDAC approval, following their mmRNAĀ® device. Having dual approved devices creates a tiered product strategy, with mmRNAĀ® positioned as the premium solution and VidaSleepā� as a more cost-effective alternative. This two-pronged approach allows Vivos to address different market segments within the estimated
The Medicare approval carries significant downstream implications beyond the federal program itself. Commercial payers typically follow CMS guidelines, potentially triggering a cascade of private insurance coverage decisions. This multiplier effect could dramatically increase Vivos' total addressable market.
The approval addresses a critical market need given that
For healthcare providers, the Medicare approval reduces reimbursement uncertainty, potentially accelerating adoption rates. The ability to offer multiple reimbursed treatment options positions Vivos favorably against competitors, while their focus on structural correction rather than just symptom management provides a compelling clinical differentiation in the crowded sleep therapy market.
Important Milestone Clears the Way for Millions of Medicare Beneficiaries with Sleep Apnea to Access Vivosā� Patented and FDA-Cleared Oral Appliance Treatment
LITTLETON, Colo., July 01, 2025 (GLOBE NEWSWIRE) -- Vivos Therapeutics, Inc. (NASDAQ: VVOS) (āVivosā� or the āCompanyā�), a pioneering medical technology company revolutionizing the treatment of obstructive sleep apnea (OSA) and snoring, today announced that its VidaSleepā� oral appliance, featuring Vivosā� patented and U.S. Food and Drug Administration (FDA) cleared Unilateral Bite Block technology, has been approved by the Centers for Medicare & Medicaid Services (CMS) Pricing, Data Analysis and Coding (PDAC) contractor for the treatment of mild to moderate OSA and snoring in adults.
The VidaSleepā¢Ā Device
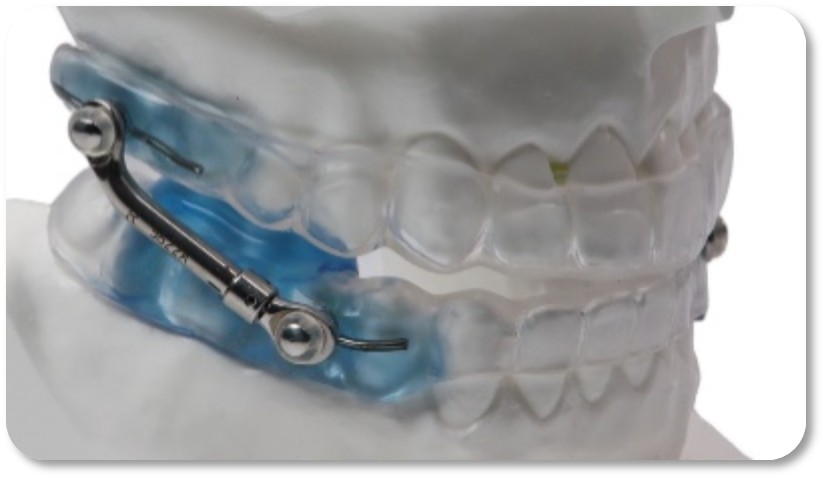
With over
With this approval, VidaSleepā� joins the Vivos mmRNAĀ® on the PDAC list of covered oral appliances, unlocking access for millions of Medicare beneficiaries seeking clinically advanced alternatives to CPAP machines or invasive surgeries. The dual-PDAC approval positions Vivos as the only company with two Medicare-covered oral appliances featuring differentiated and patented technologies designed to address the root causes of OSA while offering patients two distinct pathways and price points for therapy. The VidaSleepā� oral appliance features Vivosā� proprietary Unilateral Bite Block technology, while the mmRNA appliance is part of the FDA-cleared Vivos CARE product line for moderate to severe OSA cases in adults and children.
āThe PDAC approval of VidaSleep is another milestone achievement for Vivos, strategically positioning us to significantly augment our presence in the value-based care segment of the sleep apnea market,ā� said Kirk Huntsman, CEO and Chairman of Vivos Therapeutics. āWhile mmRNAĀ® remains our premium solution for complex cases, VidaSleep delivers positive clinical outcomes through an optimized design that maximizes accessibilityāproving that 'cost-effective' doesn't mean 'compromise.' This dual-device approach allows us to serve every tier of the estimated
Commercial & Clinical Advantages
PDAC approval not only benefits Medicare patients but also incentivizes private insurers to cover Vivosā� appliances. TheĀ VidaSleepā¢Ā device, like Vivosā� mmRNAĀ® device, is designed for use as aĀ standalone therapy or adjunct to CPAP, offering flexibility for providers and patients. VidaSleepā� offers a compelling value proposition, combining the clinical advantages of Vivosā� proprietary technology with a cost-effective price point designed to expand accessibility. Its streamlined design and efficient manufacturing process allow Vivos to deliver high-impact therapy at a budget-conscious rateāwithout compromising efficacy or qualityāincreasing opportunities for broader adoption across Medicare and commercial insurance networks while preserving strong gross margins for the healthcare providers nationwide who can now deliver these solutions with greater confidence in insurance reimbursement.
Sleep Apnea: A Silent Crisis
OSA is linked to severe comorbidities, including heart disease, stroke, and dementia, yet remains grossly underdiagnosed. Vivosā� oral appliances represent a paradigm shift by addressing the structural root causes of OSA, with peer-reviewed studies demonstrating significant improvements in airway patency and symptom resolution, including the recently announced landmark study published in the European Journal of Pediatrics.
About Vivos Therapeutics
Vivos Therapeutics, Inc. (NASDAQ: VVOS) is a medical technology company focused on developing and commercializing innovative diagnostic and treatment methods that promote sleep wellness and health for patients suffering from breathing and sleep issues such as obstructive sleep apnea (OSA) and snoring in adults. Vivosā� Complete Airway Repositioning and/or Expansion (CARE) devices are the only oral appliances cleared by the U.S. Food and Drug Administration (FDA) for adult patients diagnosed with all severity levels of OSA (including severe OSA) and moderate-to-severe OSA in children ages 6 to 17 within the FDA cleared usage for such devices.
Obstructive sleep apnea (OSA) affects over 1 billion people worldwide, yet
Vivos Therapeutics, founded in 2016 and based in Littleton, CO, is changing this. Through innovative technology, education, and collaborations with or acquisitions of functional medicine doctors, and sleep specialists, Vivos is empowering healthcare providers to more thoroughly address the complex needs of patients suffering with OSA.
Vivosā� portfolio of cutting-edge oral appliances offer a proprietary, clinically effective OSA solution that is nonsurgical, noninvasive, and nonpharmaceutical, providing hope to allow patients to Breathe New Life. For more information, visit www.vivos.com.Ā
Cautionary Note Regarding Forward-Looking Statements
This press release, including statements of the Companyās management and other parties made herein, contain āforward-looking statementsā� (as defined in Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended) concerning future events. Words such as āmayā�, āwouldā�, āshouldā�, āexpectsā�, āprojectsā�, āopportunityā�, āpotential,ā� āintendsā�, āplansā�, ābelievesā�, āanticipatesā�, āhopesā�, āestimatesā�, āgoalā�, āaimā� and variations of such words and similar expressions are intended to identify forward-looking statements. In this press release, forward-looking statements include, without limitation, those relating to the anticipated benefits to Vivos of the PDAC approval for the VidaSleepā� device as described herein. These statements involve significant known and unknown risks and are based upon several assumptions and estimates, which are inherently subject to significant uncertainties and contingencies, many of which are beyond Vivosā� control. Readers are cautioned that actual results may differ materially and adversely from those expressed or implied by such forward-looking statements. Factors that could cause actual results to differ materially include, but are not limited to: (i) the risk that Vivos may be unable to successfully implement sales, marketing and other strategies that increase revenues, (ii) the risk that some patients may not achieve the desired results from using Vivosā� products, (iii) the risk that published study data may not be predictive of results with Vivos treatment for all patients, (iv) risks associated with regulatory scrutiny of and adverse publicity in the sleep apnea diagnosis and treatment sector; (v) the risk that Vivos may be unable to secure additional financing when needed, if at all, or maintain its Nasdaq listing, (vi) market and other conditions that could impact Vivosā� business or ability to obtain financing (including whether insurance will cover the cost of Vivos treatment), and (vii) other risk factors described in Vivosā� filings with the Securities and Exchange Commission (āSECā�). Vivosā� filings can be obtained free of charge on the SECās website at www.sec.gov. Except to the extent required by law, Vivos expressly disclaims any obligations or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Vivosā� expectations with respect thereto or any change in events, conditions, or circumstances on which any statement is based.
Media Inquiries:Ā
Karla Jo HelmsĀ
JOTO PRā¢Ā�
727-777-4629Ģż
jotopr.com
A photo accompanying this announcement is available at









