Aspire Biopharma Announces Positive Top-Line Results from Clinical Trial of Investigational New Sublingual Aspirin Product for Treatment of Suspected Acute Myocardial Infarction (Heart Attack)
Aspire Biopharma (NASDAQ:ASBP) has announced positive top-line results from its clinical trial evaluating a new sublingual aspirin product for treating suspected acute myocardial infarction (heart attack). The randomized crossover bioavailability study demonstrated that the sublingual formulation achieved higher and faster plasma concentrations of acetylsalicylic acid compared to standard chewed aspirin tablets.
The trial involved six healthy subjects aged 40-65 years who received 162mg aspirin doses across three treatment periods. The sublingual delivery system offers several advantages, including rapid absorption, faster onset of action, and reduced GI irritation. This development is particularly significant as AMI affects nearly 3 million Americans annually, with 800,000 experiencing heart attacks and 300,000 resulting in deaths.
Aspire Biopharma (NASDAQ:ASBP) ha comunicato risultati positivi preliminari da uno studio clinico su un nuovo prodotto di aspirina sublinguale per il trattamento del sospetto infarto miocardico acuto. Lo studio randomizzato crossover di bio-disponibilità ha mostrato che la formulazione sublinguale raggiunge concentrazioni plasmatiche di acido acetilsalicilico più elevate e più rapide rispetto alle tradizionali compresse masticate.
La sperimentazione ha coinvolto sei soggetti sani di età compresa tra 40 e 65 anni che hanno ricevuto dosi da 162 mg di aspirina in tre periodi di trattamento. Il sistema sublinguale presenta diversi vantaggi, tra cui assorbimento rapido, insorgenza dâazione più veloce e minore irritazione gastrointestinale. Questo progresso è rilevante considerando che l'IMA colpisce quasi 3 milioni di americani ogni anno, con 800.000 infarti e 300.000 decessi.
Aspire Biopharma (NASDAQ:ASBP) ha anunciado resultados positivos preliminares de su ensayo clÃnico sobre un nuevo producto de aspirina sublingual para el tratamiento del presunto infarto agudo de miocardio. El estudio aleatorizado cruzado de biodisponibilidad demostró que la formulación sublingual alcanzó concentraciones plasmáticas de ácido acetilsalicÃlico más altas y más rápidas que las tabletas masticadas estándar.
El ensayo incluyó a seis sujetos sanos de entre 40 y 65 años que recibieron dosis de 162 mg de aspirina en tres periodos de tratamiento. El sistema sublingual ofrece varias ventajas, como absorción rápida, inicio de acción más veloz y menor irritación gastrointestinal. Este avance es especialmente relevante dado que el IAM afecta a casi 3 millones de estadounidenses al año, con 800.000 infartos y 300.000 muertes.
Aspire Biopharma (NASDAQ:ASBP)ë� ìì¬ëë ê¸ì± ì¬ê·¼ê²½ì(ì¬ì¥ë§ë¹) ì¹ë£ë¥� ìí ìë¡ì� ì¤í(íë°�) ìì¤í¼ë¦° ì íì� ëí� ìììíì� ê¸ì ì � íë¼ì� ê²°ê³¼ë¥� ë°ííìµëë¤. 무ìì� êµì°¨ ìì²´ì´ì©ë¥� ì°êµ¬ìì ì¤í ì íì íì¤ ì¹ì´ ë³µì©íë ìì¤í¼ë¦° ì ì ë³´ë¤ íì¤ ìì¸í¸ì´ë¦¬ì¤ì� ëëë¥� ë� ëê³ ë� 빨리 ëë¬íë ê²ì¼ë¡� ëíë¬ìµëë¤.
ì´ë² ìíì 40~65ì¸ì ê±´ê°í� ì±ì¸ 6ëª ì ëìì¼ë¡� 3í� ì¹ë£ 기ê°ì� ê±¸ì³ 162mg ìì¤í¼ë¦°ì� í¬ì¬í� ìíëììµëë�. ì¤í ì ë¬ ìì¤í ì ë¹ ë¥¸ í¡ì, ë� ë¹ ë¥¸ ìì© ë°í, ìì¥ ìê·¹ ê°ì ë� ì¬ë¬ ì¥ì ì� ì ê³µí©ëë�. ì� ê°ë°ì 매ë ê±°ì 300ë§� ëª ì 미êµì¸ì´ ê¸ì±ì¬ê·¼ê²½ìì� ìí¥ì� ë°ì¼ë©�, ê·¸ì¤ 80ë§� ê±´ì´ ì¬ì¥ë§ë¹ì´ê³ 30ë§� ëª ì´ ì¬ë§íë¤ë� ì ìì� ì¤ìí©ëë�.
Aspire Biopharma (NASDAQ:ASBP) a annoncé des résultats préliminaires positifs issus dâun essai clinique évaluant un nouveau produit dâaspirine sublinguale pour traiter les suspicions dâinfarctus aigu du myocarde. Lâétude randomisée en crossover de biodisponibilité a montré que la formulation sublinguale atteignait des concentrations plasmatiques dâacide acétylsalicylique plus élevées et plus rapides que les comprimés dâaspirine mâchés classiques.
Lâessai a inclus six sujets sains âgés de 40 à 65 ans ayant reçu des doses de 162 mg dâaspirine au cours de trois périodes de traitement. Le système dâadministration sublinguale présente plusieurs avantages, notamment absorption rapide, début dâaction plus rapide et moindre irritation gastro-intestinale. Cette avancée est particulièrement significative puisque lâinfarctus touche près de 3 millions dâAméricains chaque année, avec 800 000 crises et 300 000 décès.
Aspire Biopharma (NASDAQ:ASBP) hat positive Topline-Ergebnisse einer klinischen Studie zu einem neuen sublingualen Aspirin-Produkt zur Behandlung des Verdachts auf akuten Myokardinfarkt (Herzinfarkt) bekanntgegeben. Die randomisierte Crossover-Bioverfügbarkeitsstudie zeigte, dass die sublinguale Formulierung höhere und schnellere Plasmakonzentrationen von Acetylsalicylsäure erzielte als herkömmliche gekaute Aspirintabletten.
Die Studie umfasste sechs gesunde Probanden im Alter von 40â�65 Jahren, die in drei Behandlungsperioden jeweils 162 mg Aspirin erhielten. Das sublinguale Verabreichungssystem bietet mehrere Vorteile, darunter
- Clinical trial demonstrated superior drug delivery with higher and faster plasma concentrations compared to standard aspirin
- Sublingual delivery system bypasses first-pass metabolism, offering faster onset of action and reduced GI irritation
- Targets a large market with 18 million Americans living with coronary artery disease
- Product showed positive safety and tolerability profile in trial
- Small trial size of only 6 subjects limits statistical significance
- Product still requires FDA approval and further clinical trials
- Potential regulatory hurdles for accelerated approval pathway
Insights
Aspire's sublingual aspirin shows faster absorption and higher concentration than standard aspirin tablets - potentially lifesaving for heart attack patients.
Aspire Biopharma's clinical trial results for their sublingual aspirin formulation represent a significant potential advancement in acute myocardial infarction (AMI) treatment. The crossover bioavailability study demonstrated that their sublingual delivery system achieved higher and more rapid plasma concentrations of acetylsalicylic acid compared to standard chewed aspirin tablets with statistical significance (p<0.05).
The pharmacokinetic advantages of this delivery method are substantial. By bypassing first-pass metabolism in the liver, sublingual administration allows the active compound to enter the bloodstream directly through the oral mucosa's rich vascular network. This is particularly crucial in AMI treatment where minutes matter - faster antiplatelet action could potentially reduce myocardial damage and improve survival rates.
The clinical implications are profound when we consider the epidemiology: approximately 800,000 Americans experience AMI annually, resulting in 300,000 deaths. Current guidelines recommend immediate aspirin administration, preferably chewed for faster onset. However, Aspire's sublingual formulation may provide even more rapid therapeutic effects while potentially reducing gastrointestinal side effects common with oral aspirin.
The safety profile appears promising, with the drug being well-tolerated in this small initial study. While the trial was limited to only six participants, the crossover design strengthens the reliability of the comparative data despite the small sample size. The company's plan to pursue accelerated approval makes strategic sense given the potential public health benefit, though larger studies will certainly be required to confirm these preliminary findings.
Aspire's positive trial results for sublingual aspirin create significant commercial opportunity in the massive heart attack treatment market.
Aspire Biopharma's clinical trial results demonstrate impressive pharmacokinetic advantages for their sublingual aspirin formulation that could translate into substantial market opportunity. The statistically significant superiority in absorption rate and plasma concentration compared to conventional aspirin tablets represents a meaningful clinical differentiation in the critical AMI treatment window.
From a commercial perspective, this development is particularly noteworthy as it applies new drug delivery technology to aspirin - an established, trusted therapeutic with decades of safety data. This approach significantly reduces development risk compared to novel compounds while potentially creating a premium-priced, proprietary treatment option in a massive market. With 18 million Americans living with coronary artery disease, the addressable market is substantial.
The company's strategy to pursue accelerated FDA approval pathway is logical given the urgent medical need and extensive existing safety data on aspirin. If successful, this could dramatically shorten the timeline to commercialization. The patent-pending drug delivery technology mentioned suggests Aspire has established intellectual property protection, creating potential barriers to competition.
What's particularly valuable is the potential for this product to become standard of care in emergency settings - ambulances, emergency departments, and cardiac care units - creating an institutional market segment with more predictable adoption patterns than consumer markets. Additionally, the reduced risk of gastrointestinal irritation could position this as preferred for ongoing maintenance therapy in high-risk cardiac patients, expanding the commercial opportunity beyond just acute events.
According to the , Acute myocardial infarction (AMI) is a major cause of death, affecting nearly 3 million Americans each year and resulting in over a million deaths
Trial demonstrates dramatically higher and more rapid therapeutic impact compared to standard chewed aspirin tablets in clinical trial
Aspire's sublingual aspirin was safe and well-tolerated
Aspire plans to review clinical trial results with the FDA to enable a potential regulatory submission for accelerated approval
ESTERO, FL / / August 18, 2025 / Aspire Biopharma Holdings, Inc. (Nasdaq:ASBP) ("Aspire" or the "Company"), developer of a multi-faceted patent-pending drug delivery technology, today announced positive top-line data from its recent randomized, crossover bioavailability trial to assess the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of Aspire's investigational new sublingual aspirin product compared to chewed uncoated aspirin tablets in healthy adults. Pharmacokinetics is the term that describes the four stages of absorption, distribution, metabolism, and excretion of drugs. Pharmacodynamics (sometimes described as what a drug does to the body) is the study of the biochemical, physiologic, and molecular effects of drugs on the body.
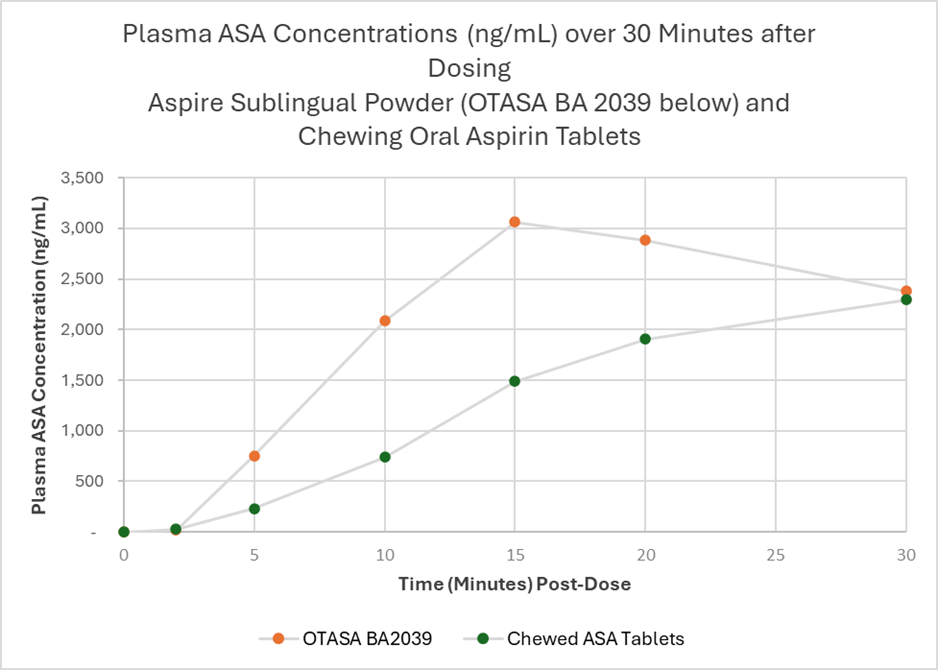
The Aspire sublingual aspirin product produced higher and more rapid mean plasma concentrations of acetylsalicylic acid (ASA, the active antiplatelet form of aspirin) compared to chewed aspirin tablets (p<0.05). Significant improvement in absorption was evident within five minutes and continued throughout the first half-hour after dosing. Higher and more rapid mean plasma concentrations imply that the drug is absorbed quickly and reaches a higher concentration in the bloodstream shortly after administration. The product was also safe and well-tolerated by patients, and no adverse events were reported.
Aspire Benefits of Sublingual Aspirin Drug Delivery and Bypassing the Gut vs Standard Oral Aspirin
Rapid absorption through the blood vessels directly, bypassing first-pass metabolic processes
Faster onset of action
Sublingual route avoids exposing the drug to the harsh acidic environment of the stomach and digestive enzymes
Reduced drug-food and drug-drug interactions
Lower risk of GI irritation
Ease of administration and use in emergency situations
Clinical trial AB-101 was a randomized crossover bioavailability study of Aspire's investigational new sublingual aspirin product compared to chewed uncoated aspirin tablets. Six otherwise healthy 40 to 65-year-old subjects were administered 162 mg aspirin as a single dose in each of three treatment periods separated by two 14-day washout periods. Two different investigational sublingual powder and granule formulations (Aspire Biopharma) and chewed uncoated oral aspirin tablets (Bayer) were studied. The primary objective of the clinical trial was to evaluate the bioavailability of ASA in plasma over eight hours after dosing.
"We are extremely pleased to report these highly positive results for our sublingual aspirin formulation, providing an important validation of our drug delivery technology," said Kraig Higginson, Chief Executive Officer of Aspire. "The effectiveness of aspirin in treating a heart attack is dependent on the time it takes to deliver ASA into the bloodstream. The ability to achieve higher and more rapid ASA concentrations for patients with suspected acute MI could save lives. We would like to thank the patients, investigators and their staff for participating in the trial, and we look forward to working with regulatory agencies to discuss next steps as we work to advance this innovative therapy and improve the treatment options for patients with suspected acute MI."
Aspire is developing its investigational sublingual aspirin product for treatment of suspected acute myocardial infarction (AMI, blockage of blood flow to heart muscle causing damage or death of heart tissue - commonly known as a "heart attack"). There are an estimated 18 million Americans living with coronary artery disease with approximately 800,000 per year experiencing an AMI leading to 300,000 deaths.
Oral aspirin is FDA-approved for treatment of suspected AMI with the initial dose of 160-162.5 mg is administered as soon as an AMI is suspected.[i] In a large, multicenter study of aspirin, streptokinase, and the combination of aspirin and streptokinase in 17,187 patients with suspected AMI, aspirin treatment produced a 23 percent reduction in the risk of death from cardiovascular diseases within five weeks.[ii] Clinical practice guidelines recommend that aspirin be initiated as soon as possible with the initial dose chewed, when possible, to achieve faster onset of antiplatelet action.[iii]
Aspire's sublingual aspirin is an investigational new drug and has not been approved for marketing by FDA or any other government regulatory authority.
About Aspire Biopharma, Inc.
Aspire Biopharma has developed a patent-pending sublingual delivery technology that can deliver drugs, nutraceuticals and supplements to the body rapidly and precisely. This allows for greater effectiveness and reduced side effects by going directly to the bloodstream and avoiding the gastrointestinal tract. Aspire Biopharma's delivery technology can be applied to many different active pharmaceutical ingredients (APIs) and other bioactive substances, spanning both small and large molecule therapeutics, nutraceuticals and supplements.
For more information, please visit
Safe Harbor Statement
This press release contains "forward-looking statements" within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended, which are intended to be covered by the "safe harbor" provisions created by those laws. Aspire's forward-looking statements include, but are not limited to, statements regarding our or our management team's expectations, hopes, beliefs, intentions or strategies regarding our future operations. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words "anticipate," "believe," "contemplate," "continue," "estimate," "expect," "intends," "may," "might," "plan," "possible," "potential," "predict," "project," "should," "will," "would," and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. These forward-looking statements represent our views as of the date of this press release and involve a number of judgments, risks and uncertainties. We anticipate that subsequent events and developments will cause our views to change. We undertake no obligation to update forward-looking statements to reflect events or circumstances after the date they were made, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. Accordingly, forward-looking statements should not be relied upon as representing our views as of any subsequent date. As a result of a number of known and unknown risks and uncertainties, our actual results or performance may be materially different from those expressed or implied by these forward-looking statements. Some factors that could cause actual results to differ include general market conditions, whether clinical trials demonstrate the efficacy and safety of our drug candidates to the satisfaction of regulatory authorities, or do not otherwise produce positive results which may cause us to incur additional costs or experience delays in completing, or ultimately be unable to complete the development and commercialization of our drug candidates; the clinical results for our drug candidates, which may not support further development or marketing approval; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials and marketing approval; our ability to achieve commercial success for our drug candidates, if approved; our limited operating history and our ability to obtain additional funding for operations and to complete the development and commercialization of our drug candidates; and other risks and uncertainties set forth in "Risk Factors" in our most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q. In addition, statements that "we believe" and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this press release, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and you are cautioned not to rely unduly upon these statements. All information in this press release is as of the date of this press release. The information contained in any website referenced herein is not, and shall not be deemed to be, part of or incorporated into this press release.
[i] U.S. Food and Drug Administration. (2022, October 14). Final Administrative Order (OTC000027): Internal Analgesic, Antipyretic, and Antirheumatic Drug Products for Over-the-Counter Human Use.
[ii] ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988; 2:349-60.
[iii] Rao SV, O'Donoghue ML, Ruel M, et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2025; 151:e771-e862.
Aspire Biopharma Holdings, Inc.
Contact
PCG Advisory
Kevin McGrath
+1-646-418-7002
[email protected]
SOURCE: Aspire Biopharma Holdings, Inc.
View the original on ACCESS Newswire








