Cardiff Oncology Announces Positive Data from Ongoing Randomized Phase 2 First-line RAS-mutated mCRC Clinical Trial (CRDF-004)
Cardiff Oncology (Nasdaq: CRDF) announced positive data from its Phase 2 clinical trial evaluating onvansertib combined with standard-of-care (SoC) for first-line RAS-mutated metastatic colorectal cancer (mCRC). The trial demonstrated a 49% confirmed objective response rate (ORR) in the 30mg onvansertib arm versus 30% in the control arm.
Key highlights include:
- Dose-dependent response across all endpoints including ORR, early tumor shrinkage, and depth of response
- Early PFS data showing favorable trend for 30mg onvansertib dose
- Well-tolerated safety profile with neutropenia as the most common treatment-emergent adverse event
The company plans to engage with FDA regarding the registrational CRDF-005 trial and expects to provide a program update by Q1 2026.
Cardiff Oncology (Nasdaq: CRDF) ha annunciato dati positivi dal suo studio clinico di Fase 2 che valuta onvansertib in combinazione con la terapia standard (SoC) per il trattamento di prima linea del carcinoma colorettale metastatico (mCRC) con mutazione RAS. Lo studio ha mostrato un tasso di risposta obiettiva confermata (ORR) del 49% nel gruppo trattato con 30mg di onvansertib, rispetto al 30% nel gruppo di controllo.
Punti salienti includono:
- Risposta dipendente dalla dose su tutti i parametri, inclusi ORR, riduzione precoce del tumore e profondità della risposta
- Dati preliminari di sopravvivenza libera da progressione (PFS) che mostrano una tendenza favorevole per la dose di 30mg di onvansertib
- Profilo di sicurezza ben tollerato, con neutropenia come evento avverso emergente da trattamento più comune
L'azienda prevede di interagire con la FDA riguardo allo studio registrativo CRDF-005 e si aspetta di fornire un aggiornamento sul programma entro il primo trimestre del 2026.
Cardiff Oncology (Nasdaq: CRDF) anunció datos positivos de su ensayo clÃnico de Fase 2 que evalúa onvansertib combinado con el tratamiento estándar (SoC) para el cáncer colorrectal metastásico (mCRC) mutado en RAS en primera lÃnea. El ensayo demostró una tasa de respuesta objetiva confirmada (ORR) del 49% en el grupo con 30mg de onvansertib frente al 30% en el grupo control.
Aspectos destacados incluyen:
- Respuesta dependiente de la dosis en todos los criterios, incluyendo ORR, reducción temprana del tumor y profundidad de la respuesta
- Datos iniciales de supervivencia libre de progresión (PFS) que muestran una tendencia favorable para la dosis de 30mg de onvansertib
- Perfil de seguridad bien tolerado, con neutropenia como el evento adverso emergente más común
La compañÃa planea dialogar con la FDA sobre el ensayo registracional CRDF-005 y espera proporcionar una actualización del programa para el primer trimestre de 2026.
Cardiff Oncology (ëì¤ë�: CRDF)ë� 1ì°� RAS ëì°ë³ì� ì ì´ì� ëì¥ì(mCRC)ì� ëí� ì¨ë°ìí°ë¸�(onvansertib)ì íì¤ ì¹ë£(SoC)ë¥� ë³ì©í� 2ì� ìììíìì ê¸ì ì ì¸ ë°ì´í°ë¥¼ ë°ííìµëë¤. ìí ê²°ê³¼ 30mg ì¨ë°ìí°ë¸� í¬ì¬êµ°ìì� íì¸ë� ê°ê´ì � ë°ìë¥�(ORR)ì� 49%ë¡� ëì¡°êµ°ì� 30%ì� ë¹í´ ëê² ëíë¬ìµëë¤.
주ì ë´ì©ì ë¤ìê³� ê°ìµëë¤:
- ORR, ì´ê¸° ì¢ ì ì¶ì ë°� ë°ì ê¹ì´ë¥� í¬í¨í� 모ë íê° ì§íìì� ì©ë ìì¡´ì � ë°ì
- 30mg ì¨ë°ìí°ë¸� ì©ëìì ì 리í� ê²½í¥ì� ë³´ì´ë� ì´ê¸° 무ì§í� ìì¡´ 기ê°(PFS) ë°ì´í�
- ê°ì� íí ì¹ë£ ë°ì ë¶ìì©ì í¸ì¤êµ� ê°ìì¦ì´ë©�, ì ë°ì ì¼ë¡� ë´ì½ì±ì´ ì¢ì ìì ì� íë¡í�
íì¬ë� ë±ë¡ ìììí CRDF-005ì� ëí� FDAì íìí� ê³íì´ë©° 2026ë � 1ë¶ê¸°ê¹ì§ íë¡ê·¸ë¨ ì ë°ì´í¸ë¥� ì ê³µí� ìì ì ëë�.
Cardiff Oncology (Nasdaq : CRDF) a annoncé des données positives issues de son essai clinique de phase 2 évaluant l'onvansertib en association avec le traitement standard (SoC) pour le cancer colorectal métastatique (mCRC) muté RAS en première ligne. L'essai a démontré un taux de réponse objective confirmée (ORR) de 49% dans le groupe recevant 30 mg d'onvansertib contre 30% dans le groupe contrôle.
Les points clés incluent :
- Réponse dépendante de la dose sur tous les critères, y compris l'ORR, la réduction précoce de la tumeur et la profondeur de la réponse
- Données précoces de survie sans progression (PFS) montrant une tendance favorable pour la dose de 30 mg d'onvansertib
- Profil de sécurité bien toléré, la neutropénie étant l'événement indésirable lié au traitement le plus fréquent
L'entreprise prévoit d'engager des discussions avec la FDA concernant l'essai d'enregistrement CRDF-005 et s'attend à fournir une mise à jour du programme d'ici le premier trimestre 2026.
Cardiff Oncology (Nasdaq: CRDF) gab positive Daten aus seiner Phase-2-Studie bekannt, in der Onvansertib in Kombination mit der Standardtherapie (SoC) für die Erstlinienbehandlung von RAS-mutiertem metastasiertem kolorektalem Krebs (mCRC) untersucht wurde. Die Studie zeigte eine bestätigte objektive Ansprechrate (ORR) von 49% in der 30mg Onvansertib-Gruppe gegenüber 30% in der Kontrollgruppe.
Wichtige Highlights umfassen:
- Dosisabhängige Reaktion über alle Endpunkte hinweg, einschlieÃlich ORR, frühe Tumorverkleinerung und Ansprechvertiefung
- Frühe PFS-Daten, die einen günstigen Trend für die 30mg Onvansertib-Dosis zeigen
- Gut verträgliches Sicherheitsprofil mit Neutropenie als häufigstem behandlungsbedingtem unerwünschtem Ereignis
Das Unternehmen plant, mit der FDA bezüglich der Zulassungsstudie CRDF-005 in Kontakt zu treten und erwartet, bis zum ersten Quartal 2026 ein Update zum Programm zu geben.
- 49% confirmed ORR in 30mg arm vs 30% in control arm, showing 19% improvement
- Dose-dependent positive response observed across all efficacy endpoints
- Early PFS data shows favorable trend for 30mg dose
- Well-tolerated safety profile with no major or unexpected toxicities
- 46% confirmed ORR at 6-months for 30mg dose vs 22% for control arm
- Median PFS has not yet been reached
- Neutropenia reported as most common treatment-emergent adverse event
- Full trial results still pending with database not yet locked
Insights
Cardiff Oncology's onvansertib shows promising 49% response rate vs 30% for standard care in RAS-mutated colorectal cancer trial.
Cardiff Oncology's Phase 2 trial results for onvansertib represent a significant development in RAS-mutated metastatic colorectal cancer (mCRC) treatment. The data shows a dose-dependent response with the 30mg arm achieving a 49% confirmed objective response rate (ORR) versus 30% in the control arm - a substantial 19% improvement. More importantly, the confirmed ORR at 6-months was 46% for the 30mg arm compared to just 22% for standard-of-care, suggesting more durable responses.
The trial design was methodologically sound, incorporating BICR (blinded independent central review) to minimize bias, and included meaningful endpoints like early tumor shrinkage and depth of response, which are established predictors of progression-free survival in first-line mCRC.
The early progression-free survival (PFS) data showing a trend favoring the 30mg dose is particularly promising, with separation of PFS curves visible at just 6 months of follow-up. The spider plots indicating deeper tumor regression in the 30mg arm further support the drug's potential efficacy.
From a safety perspective, the drug appears well-tolerated with no major or unexpected toxicities. The most common adverse event was neutropenia, which is manageable and expected with many cancer therapies.
This data positions onvansertib as a potential practice-changing therapy for RAS-mutated mCRC, a patient population with historically limited treatment options since RAS mutations typically confer resistance to anti-EGFR therapies. The company's plan to engage with the FDA about a registrational trial (CRDF-005) suggests they believe the data is strong enough to support moving toward potential approval.
Cardiff's onvansertib shows impressive 19% ORR improvement in colorectal cancer trial, positioning it for potential regulatory advancement.
Cardiff Oncology's latest clinical data represents a significant inflection point in their development of onvansertib, a PLK1 inhibitor targeting RAS-mutated metastatic colorectal cancer. The 19% improvement in confirmed objective response rate (ORR) in the 30mg dose arm (49% vs. 30% in control) is clinically meaningful in this difficult-to-treat population.
Particularly compelling is the dose-dependent response pattern observed across multiple efficacy metrics, which provides internal validation of the drug's mechanism and strengthens the case for the selected dosing regimen. The separation between the 20mg dose (42% ORR) and 30mg dose (49% ORR) suggests optimal dosing has been identified.
The 6-month confirmed ORR of 46% in the 30mg arm versus 22% in the control arm (a 24% difference) demonstrates both the magnitude and durability of treatment effect. This is reinforced by the early PFS curve separation, suggesting a potential survival benefit that could be confirmed with longer follow-up.
From a regulatory perspective, these results position Cardiff well for discussions with the FDA regarding their planned registrational CRDF-005 trial. The company's announcement that they'll provide an update on their first-line mCRC program by Q1 2026 indicates confidence in their regulatory strategy.
The safety profile appears manageable with neutropenia as the most common serious adverse event, consistent with many cancer treatments. This favorable benefit-risk profile is critical for regulatory considerations and eventual commercial adoption if approved.
With these results, Cardiff is addressing a significant unmet need in RAS-mutated mCRC, which represents approximately 50% of colorectal cancer cases and has historically had limited targeted treatment options.
â� Trial demonstrates
â� Early PFS data show a trend favoring 30mg onvansertib dose arm vs. control arm â�
â� Onvansertib continues to be well-tolerated and demonstrates a dose dependent response for all endpoints including ORR, early tumor shrinkage and depth of response â�
â� Company will hold a conference call today at 4:30 p.m. ET / 1:30 p.m. PT â�
SAN DIEGO, July 29, 2025 (GLOBE NEWSWIRE) -- Cardiff Oncology, Inc. (Nasdaq: CRDF), a clinical-stage biotechnology company leveraging PLK1 inhibition to develop novel therapies across a range of cancers, today announced positive data from the ongoing CRDF-004, a randomized, Phase 2 clinical trial evaluating onvansertib in combination with standard-of-care (SoC) in patients with first-line RAS-mutated metastatic colorectal cancer (mCRC). Efficacy data represents intent-to-treat patients as of a July 8, 2025 data cut-off, and is determined by blinded, independent central review (BICR) of each patientâs tumor scans.
âWe are highly encouraged by the
Trial Design
The CRDF-004 phase 2 trial enrolled patients with mCRC who have a documented KRAS or NRAS mutation. Onvansertib is added to SoC consisting of FOLFIRI plus bevacizumab or FOLFOX plus bevacizumab. Patients were randomized to one of six arms including 20mg of onvansertib plus SoC, 30mg of onvansertib plus SoC, or SoC alone. The primary endpoint is objective response rate (ORR), and the secondary endpoints include progression-free survival (PFS), duration of response (DOR) and safety. Additional prespecified endpoints include early tumor shrinkage (ETS), defined as a â�
Efficacy Data
Efficacy data in the intent-to-treat population (ITT) from the CRDF-004 clinical trial, as of the July 8, 2025 data cut-off, are shown below.
| Ìý | Control Arm (SoC alone) (n=37) | 20mg dose of onvansertib + SoCÌý (n=36) | 30mg dose of onvansertib + SoCÌý (n=37) |
| Confirmed ORRa | (11 of 37) | (15 of 36) | (18 of 37) |
| Confirmed ORR at 6-monthsa | (8 of 37) | (12 of 36) | (17 of 37) |
| ORRb | (16 of 37) | (18 of 36) | (22 of 37) |
aConfirmed Objective Response Rate (ORR) per RECIST v1.1 includes those patients who had a complete response (CR) or partial response (PR) confirmed by repeat imaging â�4 weeks after response criteria first met. bORR per RECIST v1.1 includes confirmed CRs/PRs and unconfirmed PRs who were still on treatment and may yet be confirmed
Spider Plots, displaying the change in tumor size from baseline for each patient over time, demonstrate deeper responses in patients receiving the 30mg dose of onvansertib in combination with the SoC compared to both the control arm and 20mg dose of onvansertib arm.
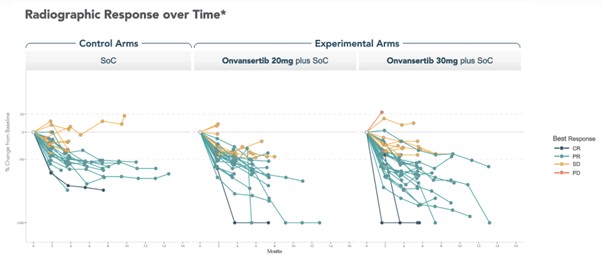
Note: Radiographic response was determined per RECIST 1.1 by blinded independent central review. Spider plot reflects data as of July 8, 2025 from an ongoing trial and unlocked database.
Progression-free Survival (PFS) Data
Both the 20mg and 30mg onvansertib arms demonstrated an early separation of the PFS curves compared to the control arm at a median follow up time of 6 months. While the median PFS has not been reached, there was a dose dependent effect in favor of the 30mg onvansertib dose.
Safety and Tolerability
The safety analysis was conducted for the 104 patients who were dosed in the trial. Onvansertib in combination with chemo/bevacizumab was well-tolerated and there were no major or unexpected toxicities observed. Grade 3 or higher adverse events were infrequent, with neutropenia being the most common treatment-emergent adverse event associated with onvansertib.
âWe are highly encouraged by the strength of our data which achieves the key objectives we set for the trial, and positions us to engage in discussions with the FDA as we advance toward our registrational CRDF-005 trial,â� said Mark Erlander, Chief Executive Officer of Cardiff Oncology. âLooking ahead, we are optimistic about onvansertibâs potential to redefine the first-line treatment for RAS-mutated mCRC and will provide an update on our first-line mCRC program by Q1 2026.â�
Upcoming expected milestones
- Update on first-line mCRC program expected by 1Q 2026
Conference Call and Webcast
Cardiff Oncology will host a conference call and live webcast at 4:30 p.m. ET / 1:30 p.m. PT on July 29, 2025. Individuals interested in listening to the live conference call may do so by using the webcast link in the "" section of the company's website. A webcast replay will be available in the investor relations section on the company's website following the completion of the call.
About Cardiff Oncology, Inc.
Cardiff Oncology is a clinical-stage biotechnology company leveraging PLK1 inhibition, a well-validated oncology drug target, to develop novel therapies across a range of cancers. The Company's lead asset is onvansertib, a PLK1 inhibitor being evaluated in combination with standard of care (SoC) therapeutics in clinical programs targeting indications such asÌýRAS-mutated metastatic colorectal cancer (mCRC), as well as in ongoing and planned investigator-initiated trials in metastatic pancreatic ductal adenocarcinoma (mPDAC), small cell lung cancer (SCLC) and triple negative breast cancer (TNBC). These programs and the Company's broader development strategy are designed to target tumor vulnerabilities in order to overcome treatment resistance and deliver superior clinical benefit compared to the SoC alone. For more information, please visitÌý.
References
- Cremolini, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann Oncol. 2015;26(6):1188â�1194. doi:Ìý10.1093/annonc/mdv112
- Piessevaux, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2013 Oct 20;31(30):3764-75. doi: 10.1200/JCO.2012.42.8532
- Bando H, et al. Associations between early tumor shrinkage/depth of response and survival from the ARCAD database. JNCI Cancer Spectr. 2025 Apr 30;9(3):pkaf042. doi: 10.1093/jncics/pkaf042
Forward-Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. These statements may be identified using words such as "anticipate," "believe," "forecast," "estimated" and "intend" or other similar terms or expressions that concern Cardiff Oncology's expectations, strategy, plans or intentions. These forward-looking statements are based on Cardiff Oncology's current expectations and actual results could differ materially. There are several factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results; our clinical trials may be suspended or discontinued due to unexpected side effects or other safety risks that could preclude approval of our product candidate; results of preclinical studies or clinical trials for our product candidate could be unfavorable or delayed; our need for additional financing; risks related to business interruptions, including the outbreak of COVID-19 coronavirus and cyber-attacks on our information technology infrastructure, which could seriously harm our financial condition and increase our costs and expenses; uncertainties of government or third party payer reimbursement; dependence on key personnel; limited experience in marketing and sales; substantial competition; uncertainties of patent protection and litigation; dependence upon third parties; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations. There are no guarantees that our product candidate will be utilized or prove to be commercially successful. Additionally, there are no guarantees that future clinical trials will be completed or successful or that our product candidate will receive regulatory approval for any indication or prove to be commercially successful. Investors should read the risk factors set forth in Cardiff Oncology's Form 10-K for the year ended December 31, 2024, and other periodic reports filed with the Securities and Exchange Commission. While the list of factors presented here is considered representative, no such list should be considered to be a complete statement of all potential risks and uncertainties. Unlisted factors may present significant additional obstacles to the realization of forward-looking statements. Forward-looking statements included herein are made as of the date hereof, and Cardiff Oncology does not undertake any obligation to update publicly such statements to reflect subsequent events or circumstances.
Cardiff Oncology Contact:
James Levine
Chief Financial Officer
858-952-7670
Investor Contact:
Kiki Patel, PharmD
Gilmartin Group
332-895-3225
Media Contact:
Meghan Bianco
Taft Communications, a division of RF|Binder
609-544-5446
A photo accompanying this announcement is available at








