BriaCell Reports Complete and Sustained Resolution of Brain Metastasis and Sustained Regression of Orbital Metastasis in “Eye-Bulging�� Breast Cancer Patient
BriaCell Therapeutics (NASDAQ: BCTX) has reported significant progress in its Phase 2 study of Bria-IMT�� immunotherapy for advanced metastatic breast cancer. A patient who had previously failed 8 prior treatment regimens has shown remarkable results after 18+ months of treatment, including complete resolution of temporal lobe brain metastasis and continued reduction of orbital tumor that had caused eye bulging.
The patient has completed 29 treatment cycles over 21 months, with serial imaging at 8, 11, and 20 months confirming no detectable disease in the right temporal lobe and continued response in the orbital lesion. Tumor markers have remained significantly reduced from baseline, supporting the sustained radiologic response.
BriaCell Therapeutics (NASDAQ: BCTX) ha annunciato importanti progressi nello studio di Fase 2 della sua immunoterapia Bria-IMT�� per il carcinoma mammario metastatico avanzato. Un paziente, che aveva fallito 8 precedenti regimi terapeutici, ha mostrato risultati notevoli dopo più di 18 mesi di trattamento, inclusa la completa risoluzione della metastasi cerebrale al lobo temporale e la continua riduzione del tumore orbitale che causava protrusione oculare.
Il paziente ha completato 29 cicli di trattamento in un periodo di 21 mesi, con immagini seriali a 8, 11 e 20 mesi che hanno confermato l'assenza di malattia rilevabile nel lobo temporale destro e una risposta persistente nella lesione orbitale. I marcatori tumorali sono rimasti significativamente ridotti rispetto al basale, a sostegno della risposta radiologica duratura.
BriaCell Therapeutics (NASDAQ: BCTX) ha informado avances significativos en su estudio de Fase 2 de la inmunoterapia Bria-IMT�� para el cáncer de mama metastásico avanzado. Un paciente que había fallado en 8 regímenes de tratamiento previos ha mostrado resultados notables después de más de 18 meses de tratamiento, incluyendo la resolución completa de la metástasis cerebral en el lóbulo temporal y la reducción continua del tumor orbital que causaba protrusión ocular.
El paciente ha completado 29 ciclos de tratamiento durante 21 meses, con imágenes seriadas a los 8, 11 y 20 meses que confirman la ausencia de enfermedad detectable en el lóbulo temporal derecho y una respuesta continua en la lesión orbital. Los marcadores tumorales se han mantenido significativamente reducidos respecto al inicio, respaldando la respuesta radiológica sostenida.
BriaCell Therapeutics (NASDAQ: BCTX)�� 진행�� 전이�� 유방암에 대�� Bria-IMT�� 면역치료제의 2�� 임상시험에서 중요�� 진전�� 보고했습니다. 이전�� 8가지 치료 요법�� 실패�� 환자가 18개월 이상 치료 �� 측두�� �� 전이�� 완전 소실�� 안구 돌출�� 유발했던 안와 종양�� 지속적 감소�� 포함�� 뛰어�� 결과�� 보였습니��.
�� 환자�� 21개월 동안 29�� 치료 주기�� 완료했으��, 8, 11, 20개월 시점�� 연속 영상 검사에�� 우측 측두엽에 감지 가능한 질병�� 없고 안와 병변에서 지속적�� 반응�� 확인되었습니��. 종양 표지자는 초기 대�� 크게 감소하여 지속적�� 영상학적 반응�� 뒷받침합니다.
BriaCell Therapeutics (NASDAQ : BCTX) a annoncé des progrès significatifs dans son étude de phase 2 sur l'immunothérapie Bria-IMT�� pour le cancer du sein métastatique avancé. Un patient ayant échoué à 8 traitements antérieurs a présenté des résultats remarquables après plus de 18 mois de traitement, incluant la résolution complète de la métastase cérébrale du lobe temporal et la réduction continue de la tumeur orbitale responsable d'une protrusion de l'œil.
Le patient a terminé 29 cycles de traitement sur une période de 21 mois, avec des imageries successives à 8, 11 et 20 mois confirmant l'absence de maladie détectable dans le lobe temporal droit et une réponse continue de la lésion orbitale. Les marqueurs tumoraux sont restés significativement réduits par rapport au départ, soutenant la réponse radiologique durable.
BriaCell Therapeutics (NASDAQ: BCTX) hat bedeutende Fortschritte in seiner Phase-2-Studie zur Bria-IMT�� Immuntherapie bei fortgeschrittenem metastasiertem Brustkrebs gemeldet. Ein Patient, der zuvor 8 vorherige Behandlungsregime nicht erfolgreich durchlaufen hatte, zeigt nach ü����� 18 Monaten Behandlung bemerkenswerte Ergebnisse, darunter die vollständige Rückbildung der temporalen Hirnlappenmetastase und eine anhaltende Verringerung des orbitären Tumors, der das Hervortreten des Auges verursacht hatte.
Der Patient hat 29 Behandlungszyklen ü����� 21 Monate abgeschlossen, wobei serielle Bildgebungen nach 8, 11 und 20 Monaten bestätigten, dass im rechten Temporallappen keine nachweisbare Erkrankung mehr vorliegt und die orbitale Läsion weiterhin anspricht. Die Tumormarker blieben im Vergleich zum Ausgangswert deutlich reduziert, was die anhaltende radiologische Reaktion unterstützt.
- None.
- Study still in Phase 2, requiring further validation
- Results based on single patient case study
Insights
BriaCell reports remarkable sustained tumor regression in a heavily pre-treated breast cancer patient, demonstrating potential clinical efficacy against brain metastases.
The updated results from BriaCell's Phase 2 study represent a significant clinical milestone. The patient's complete resolution of temporal lobe brain metastasis and continued orbital tumor reduction after more than 18 months of treatment is particularly noteworthy given the patient had previously failed 8 prior treatment regimens, including an antibody-drug conjugate (ADC) therapy.
Brain metastases present one of the most challenging treatment scenarios in breast cancer. Current therapeutic options for brain metastases are limited, with poor prognosis typically measured in months. The complete resolution of brain metastasis sustained for 18+ months without recurrence is therefore quite remarkable in this clinical context.
The orbital metastasis response is equally significant. Orbital metastases are rare (occurring in
The 29 treatment cycles completed over 21 months also indicate a manageable safety profile, essential for any maintenance therapy. The sustained reduction in tumor markers provides biochemical confirmation supporting the radiological findings.
This represents a single-patient case report from an ongoing Phase 2 trial, so broader efficacy conclusions require caution. However, durable responses in a patient with such treatment-refractory disease suggest Bria-IMT's immunotherapeutic approach may offer meaningful clinical benefit in a patient population with limited options and poor prognosis.
- [IMAGES BELOW] Sustained complete resolution of temporal lobe brain metastasis and continued orbital tumor reduction after >18 months of treatment
- “Eye bulging�� metastatic breast cancer patient had failed 8 prior regimens, including antibody-drug conjugate (ADC) therapy
- Patient remains on BriaCell’s Phase 2 study with 29 treatment cycles completed
PHILADELPHIA and VANCOUVER, British Columbia, July 10, 2025 (GLOBE NEWSWIRE) -- BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW, BCTXZ) (TSX: BCT) (“BriaCell�� or the “Company��), a clinical-stage biotechnology company developing novel immunotherapies to transform cancer care today announced updated results from its ongoing Phase 2 study of Bria-IMT�� in combination with check point inhibitor in patients with advanced metastatic breast cancer (MBC).
BriaCell is pleased to report the sustained complete resolution of temporal lobe brain metastasis and continued orbital (behind the eye) tumor reduction after > 18 months of treatment in the Phase 2 study. The metastatic tumor initially caused proptosis –a visibly bulging of the eye. As previously reported, the heavily pre-treated MBC patient had demonstrated a and a significant response in a right orbital metastasis at 8 months, then at 11 months. The patient has now maintained both responses for more than 18 months, with no evidence of brain tumor recurrence and ongoing tumor shrinkage in the orbital lesion.
Figure 1: Bria-IMT treatment resulted in complete resolution of the right temporal lobe lesion and continued regression of the right orbital (behind the eye) tumor. The right temporal lobe lesion is no longer detectable on the images taken at 8 months (May 2024), 11 months (Aug 2024), and 20 months (May 2025).
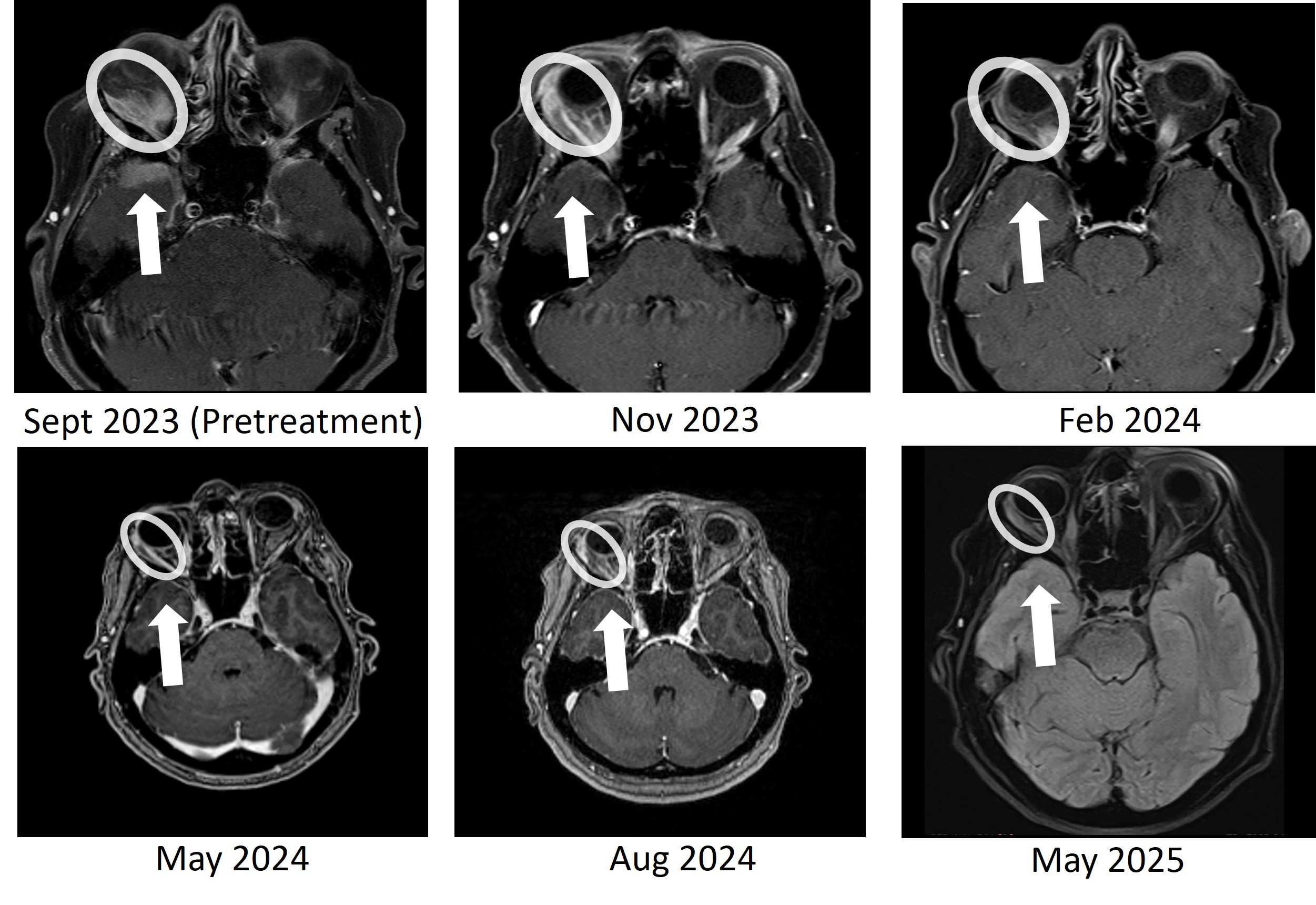
This patient had failed eight prior treatment regimens, including an antibody-drug conjugate (ADC), before initiating therapy with Bria-IMT plus checkpoint inhibition. She has now completed 29 treatment cycles and has been on BriaCell’s Phase 2 study for over 21 months. Serial imaging at 8, 11, and now 20 months have confirmed no detectable disease in the right temporal lobe, along with continued response in the orbital lesion. Additionally, the patient’s tumor markers have remained markedly reduced from baseline, further supporting the sustained radiologic response.
“These encouraging results continue to suggest that our novel Bria-IMT regimen may provide durable immunotherapeutic benefit in late-stage breast cancer patients with brain metastases who have exhausted other options,�� stated Dr. William V. Williams, BriaCell’s President & CEO. “The long-term response observed in this patient reinforces the potential of Bria-IMT to improve outcomes while maintaining a favorable tolerability profile.��
About BriaCell Therapeutics Corp.
BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at .
Safe Harbor
This press release contains “forward-looking statements�� that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as “anticipate,�� “believe,�� “contemplate,�� “could,�� “estimate,�� “expect,�� “intend,�� “seek,�� “may,�� “might,�� “plan,�� “potential,�� “predict,�� “project,�� “target,�� “aim,�� “should,�� “will,�� “would,�� or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements, including those about BriaCell’s Bria-IMT regimen bringing relief to cancer patients whose medical needs remain unmet and the Bria-IMT regimen becoming a therapeutic option for metastatic breast cancer patients, are based on BriaCell’s current expectations and are subject to inherent uncertainties, risks, and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading “Risks and Uncertainties�� in the Company’s most recent Management’s Discussion and Analysis, under the heading “Risk Factors�� in the Company’s most recent Annual Information Form, and under “Risks and Uncertainties�� in the Company’s other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Company's profiles on SEDAR+ at and on EDGAR at . Forward-looking statements contained in this announcement are made as of this date, and BriaCell Therapeutics Corp. undertakes no duty to update such information except as required under applicable law.
Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release.
Contact Information
Company Contact:
William V. Williams, MD
President & CEO
1-888-485-6340
Investor Relations Contact:
A photo accompanying this announcement is available at








